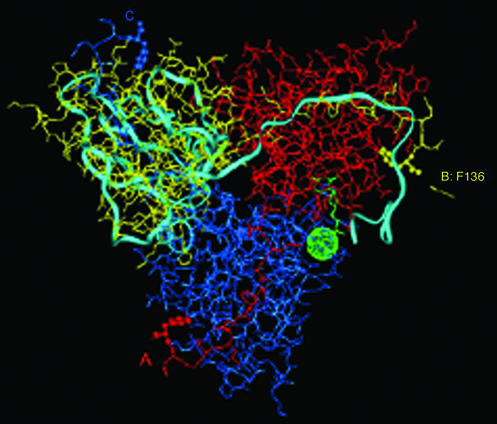
Figure 1.
X-ray determined structure of Escherichia coli dUTPase (4, 5). The subunits of the trimeric enzyme are shown in different colors: A, red; B, yellow; and C, blue. The light-blue ribbon structure illustrates one subunit of the human enzyme (3) superpositioned according to structurally equivalent residues (7). The yellow arrow identifies the ultimate C-terminal residue, Phe-136, that is observed by x-ray in the E. coli enzyme (4–6). Rendering this residue in ball-and-stick form highlights its location in each subunit of the enzyme. The dUDP inhibitor bound to the E. coli enzyme (5) and Sr2+ in the EIAV enzyme (7) in green identify the location of one of the active sites.