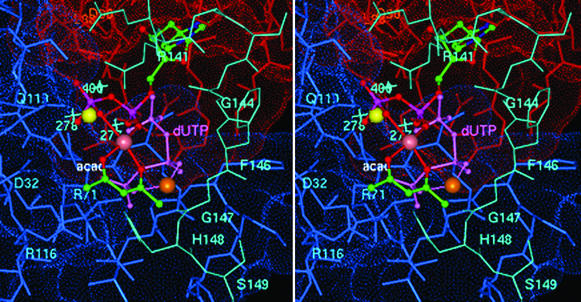
Figure 4.
Stereoview of the coordination geometry of [VO(acac)+] in the active site of E. coli dUTPase with dUDP proposed on the basis of EPR and ENDOR results. The uracil and deoxyribose moieties were superpositioned onto their counterparts of dUDP in EIAV dUTPase (7). Atoms of the acetylacetonate ligand, color-coded according to element and labeled “acac,” are shown on the front side of the metal ion with the α- and β-phosphate oxygens of dUDP coordinated to the metal ion from the back side. Both supply equatorial oxygen-donor atoms to the vanadium (rendered as a mauve sphere). Subunit A residues are red, subunit C residues are blue, and residues corresponding to the structurally disordered C-terminal fragment of the B subunit in the E. coli enzyme and modeled according to the conformation of ordered C-terminal residues of human dUTPase (ref. 3; cf. Table 1) are cyan. Also shown is the 5′-triphosphate moiety of dUTP (light purple and labeled dUTP) coordinated to the V4+ ion (orange sphere) to illustrate a possible mode of metal ion binding in the catalytically competent ternary complex and its interaction with C-terminal residues. The Sr2+ ion, as coordinated to the diphosphate moiety of dUDP in the homologous EIAV enzyme (7), is yellow. The light-blue crosses indicate the corresponding locations of three hydrogen-bonded water molecules in the active site of the human enzyme (3) nearest to the α-phosphorus of the 5′-triphosphate group. It is seen that these water molecules overlap significantly with the Sr2+ ion. The Lee–Richards solvent-accessible surface (31) of active site residues is color-coded according to subunit. Active-site residues are labeled for purposes of orientation and are color-coded according to subunit origin.