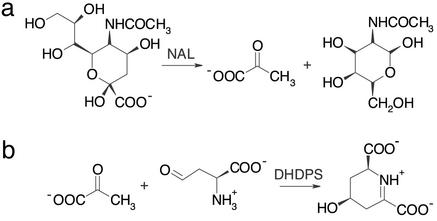
Figure 1.
Reactions catalyzed by NAL (a) and DHDPS (b), two members of the NAL subfamily of (β/α)8-barrel enzymes. The common catalytic step is a Schiff-base formation between a conserved lysine and the keto group of the respective substrate followed by the aldol condensation/cleavage reaction. In DHDPS, subsequent transimination occurs to yield the cyclic product. On the basis of NMR experiments, Blickling et al. (14) proposed that (4S)-4-hydroxy-2,3,4,5-tetrahydro-(2S)-dipicolinic acid is the immediate product of the reaction catalyzed by DHDPS. The elimination of a water molecule to yield dihydrodipicolinate is supposed to occur spontaneously after the initial product is released into the solvent.