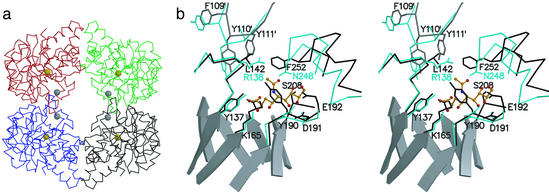
Figure 3.
The structure of NAL. (a) The NAL tetramer. Cα-traces of individual subunits are shown in black (chain A), red (chain B), blue (chain C), and green (chain D). The golden spheres are centered on the Nζ-atoms of K165, which form a Schiff-base during catalysis; the gray spheres are centered on the Cα-atoms of L142. The subunit interface discussed in this work is between chains A/D and B/C respectively. (b) Stereoview of the active center of NAL from E. coli (black) in complex with BHP (12) superimposed on DHDPS (cyan) from E. coli (13). The superposition was calculated based on the coordinates for the main-chain atoms in β5, connective loop β5/α5, and β6. For clarity, active site loops are shown as Cα-traces with selected side chains. Residues contributed from an adjacent subunit are marked (′); e.g., Y111′. β-Strands forming the central barrel in NAL are shown as ribbon models. Also shown as ball-and-stick model (orange) is the NAL substrate analog sialic acid alditol in the conformation and position as found in the structure of NAL from H. influenzae (9).