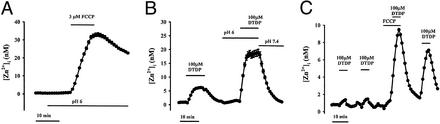
Figure 2.
Mobilization of mitochondrial and protein-bound Zn2+ pools. (A and B) Acidosis greatly enhances intracellular Zn2+ mobilization. (A) FluoZin-3-loaded cultures were exposed to FCCP (3 μM) after a 10-min preincubation in acidic (pH 6.0) buffer. Note that the [Zn2+]i rise is nearly an order of magnitude greater than that induced by an identical exposure at physiological pH (Fig. 1C; Table 1). (B) FluoZin-3-loaded cultures were exposed to the oxidizing agent, DTDP (10 min, 100 μM) at physiological pH, and after a 10-min preincubation at pH 6.0. Note the increased [Zn2+]i response at pH 6 and the rapid recovery on restoration of physiological pH. (C) Relocation of Zn2+ from mitochondria to protein-bound sites. FluoZin-3-loaded cultures were exposed to consecutive 5-min pulses of DTDP (100 μM), FCCP (3 μM), and both before a final exposure to DTDP, as indicated. Note that combined exposure to DTDP and FCCP induced far greater [Zn2+]i rises than exposure to FCCP alone, suggesting that in the absence of oxidation, Zn2+ released from mitochondria gets rapidly bound by redox-sensitive proteins. Further note the markedly increased size of the final DTDP response, indicative of a relocation of Zn2+ from the mitochondrial compartment to redox-sensitive protein pools. Traces show mean [Zn2+]i (±SEM) of 63–83 neurons, from 1 experiment representative of 5–14.