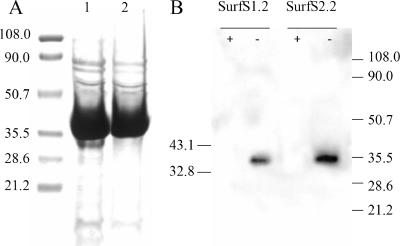
FIG. 2.
Purification of two selected scFv clones and antigen recognition in M. avium subsp. paratuberculosis cell lysate. (A) Purification of scFv His-tagged fusion proteins SurfS1.2 (lane 1) and SurfS2.2 (lane 2). scFv were expressed using the vector pAK500 in JM83 E. coli host cells for 18 h at 25°C. The cells were lysed, the supernatant collected, and the fusion protein purified by immobilized metal affinity chromatography. Approximately 1% of purified scFv from a 200-ml culture was resolved by 10% SDS-PAGE and stained with Coomassie-brilliant blue. (B) Immunoblotting of M. avium subsp. paratuberculosis lysate and detection with SurfS1.2 or SurfS2.2. Approximately 20 μg of mycobacterial proteins were either digested with proteinase K (+) or remained untreated (−) and resolved on a 10% SDS-PAGE gel, and the products were transferred to nitrocellulose and probed with peroxidase-conjugated anti-His antibodies. The figure depicts strong binding of both antibodies to a proteinase-susceptible 34-kDa determinant.