Abstract
Enterobacter sakazakii has been reported to form biofilms, but environmental conditions affecting attachment to and biofilm formation on abiotic surfaces have not been described. We did a study to determine the effects of temperature and nutrient availability on attachment and biofilm formation by E. sakazakii on stainless steel and enteral feeding tubes. Five strains grown to stationary phase in tryptic soy broth (TSB), infant formula broth (IFB), or lettuce juice broth (LJB) at 12 and 25°C were examined for the extent to which they attach to these materials. Higher populations attached at 25°C than at 12°C. Stainless steel coupons and enteral feeding tubes were immersed for 24 h at 4°C in phosphate-buffered saline suspensions (7 log CFU/ml) to facilitate the attachment of 5.33 to 5.51 and 5.03 to 5.12 log CFU/cm2, respectively, before they were immersed in TSB, IFB, or LJB, followed by incubation at 12 or 25°C for up to 10 days. Biofilms were not produced at 12°C. The number of cells of test strains increased by 1.42 to 1.67 log CFU/cm2 and 1.16 to 1.31 log CFU/cm2 in biofilms formed on stainless steel and feeding tubes, respectively, immersed in IFB at 25°C; biofilms were not formed on TSB and LJB at 25°C, indicating that nutrient availability plays a major role in processes leading to biofilm formation on the surfaces of these inert materials. These observations emphasize the importance of temperature control in reconstituted infant formula preparation and storage areas in preventing attachment and biofilm formation by E. sakazakii.
Enterobacter sakazakii is a food-borne pathogen capable of causing meningitis (4, 13, 38), sepsis (38), bacteremia (40), and necrotizing enterocolitis (55) in preterm neonates and immunocompromised adults (9, 15, 25, 30, 45). Its genetic and phenotypic diversity has been demonstrated (24, 33), and certain clusters may substantially differ in thermal tolerance (8, 58). Powdered infant formula and milk powder have been implicated as vehicles in outbreaks of E. sakazakii infections (3, 18, 37-39, 51, 55). E. sakazakii was detected in 20 of 141 (14.2%) powdered infant formulas originating from 13 countries (37). The pathogen has been isolated from various clinical sources, food processing plants, the environment (22, 27), lettuce (52), alfalfa sprouts (5), tomatoes (26), and other vegetables, cheese, minced beef, and sausage (31). Its presence in fresh produce raises the possibility of this food group serving as a vehicle of the pathogen for infections in immunocompromised adults, particularly patients in hospitals and elderly adult assisted-care facilities.
Attachment of bacterial cells to surfaces may be followed by growth, the production of exopolysaccharide, and biofilm formation (29). Biofilms have been defined as sessile communities of cells attached to a surface or to each other, usually embedded in polymeric substances produced by the bacteria (34). The attachment of microorganisms to biotic or abiotic surfaces, followed by biofilm formation, is known to enhance the resistance of cells to environmental stresses and to provide protection against sanitizers (12, 29, 41, 48). Factors affecting attachment and biofilm formation by microorganisms include nutrient availability, the pH of the surrounding medium, and the nature of the cell and abiotic surfaces (11).
E. sakazakii has been reported to be able to attach to and form biofilms on silicon, latex, polycarbonate, stainless steel, glass, and polyvinyl chloride (PVC) (23, 32). Foods such as powdered infant formula and fresh produce represent potential vehicles of E. sakazakii infections in infants and immunocompromised adults, respectively. Contact of these and other foods containing the pathogen with abiotic or biotic surfaces could result in attachment and biofilm formation. Attachment or biofilms of E. sakazakii on equipment surfaces used in formula preparation and feeding areas or in produce processing plants may increase the risk of infections to infants and immunocompromised adults, respectively. E. sakazakii colonization on the surfaces of a spoon, brush, and blender used for infant formula preparation has been documented to occur in a clinical setting where neonatal infections were reported (1, 38, 40, 51). Reuse of enteral feeding tubes and delivery bags after washing may increase the risk of microbial infections (42). Biofilm formation by pathogens on fresh fruits and vegetables during harvesting, transporting, processing, and storage has been conjectured to potentially increase the risk of food-borne diseases in individuals consuming these products (2). We have observed that E. sakazakii is able to grow on fresh-cut produce and in produce juice (28).
Removal or inactivation of pathogens on inert surfaces in infant formula preparation areas and produce processing environments by washing with water or treatment with disinfectants or sanitizers is not always achieved, possibly because cells are enmeshed in biofilms or otherwise protected against exposure to antimicrobials. Attachment and biofilm formation by E. sakazakii as affected by temperature and nutrient availability have been given only meager research attention. The objectives of the present study were to determine the growth characteristics of E. sakazakii in a rich microbiological medium, reconstituted infant formula, and lettuce juice as a produce model and characterize subsequent attachment of stationary-phase cells and biofilm formation on the surfaces of stainless steel and enteral feeding tubes as affected by temperature and nutrients provided by these media.
MATERIALS AND METHODS
Strains examined.
Five clinical strains (2855, 3231, 3234, 3290, and 3295), four strains (2871, 3270, 3437, and 3439) isolated from foods, and one strain (3396) isolated from an environmental source were examined for colony morphology on tryptic soy agar (TSA; BBL/Difco, Sparks, Md.), infant formula agar (IFA), and lettuce juice agar (LJA) (see the discussion of the preparation of media below). Cultures grown into 10 ml of tryptic soy broth (TSB; BBL/Difco) at 37°C for 24 h were streaked on TSA, IFA, and LJA and incubated at 12, 25, and 37°C for 6, 2, and 1 days, respectively. Three strains (3231, 3234, and 3396) producing colonies distinctly wet and mucoid in appearance and three strains (2855, 3295, and 3439) producing drier nonmucoid colonies were selected for determining growth curves in TSB, infant formula broth (IFB), and lettuce juice broth (LJB). Strains 3231, 3234, 3295, 3439, and 3396 were used for the attachment study; because strain 2855 showed a very different growth pattern compared to the other five strains, it was not included. For the biofilm study, E. sakazakii strain 3231, which produces mucoid colonies, and strain 3439, which produces nonmucoid colonies on IFA, were used to determined whether there are significantly different patterns of attachment or biofilm formation based, in part, on these characteristics.
Preparation of media.
Similac Neosure Advance infant formula (Ross Pediatrics, Abbott Laboratories, Columbus, Ohio) was selected to make IFB because preliminary studies showed that the development of mucoid colonies by some of the test strains was more evident on IFA prepared using this formula than on IFA prepared using four other formulas. Milk-based infant formulas such as Similac Neosure Advance are consumed more widely than are soy-based formulas, giving additional incentive for its use in attachment and biofilm studies. IFB (pH 6.6) was made by combining Similac Neosure Advance infant formula with distilled water at a 1:10 ratio (wt/vol), dissolving by heating at 50 to 60°C, and autoclaving. Some heat-labile vitamins may have been lost during autoclaving. To prepare LJB (pH 6.3), iceberg lettuce (Lactuca sativa L.) was blended in an automatic juice extractor (The Juiceman, Jr.; Trillium Health Products, J. M. Marketing, Inc., Seattle, Wash.), filtered through 16 layers of coarsely woven cheese cloth, brought to a boil, and refiltered through 16 layers of cheese cloth to remove coarse particles. TSB (pH 7.0) and TSA were prepared according to the manufacturer's directions. Powdered infant formula (100 g) and agar (BBL/Difco) (15 g) were combined with 900 ml of distilled water, heated to dissolve the contents, and autoclaved (15 min at 121°C) to make IFA. LJA was made by adding 15 g of agar to 1 liter of LJB, heated to dissolve the contents, and autoclaved. For the growth curve study, 300 ml of each broth in 500-ml capped bottles were used. For attachment and biofilm experiments, 30 ml of each broth in 50-ml centrifuge tubes was used.
Phosphate-buffered saline (PBS; pH 7.4) used to resuspend E. sakazakii cells for use in biofilm experiments was prepared by combining NaCl (8 g), KCl (0.2 g), Na2HPO4 (1.44 g), and KH2PO4 (0.24 g) with distilled water (1 liter) and autoclaving. Glass beads (425 to 600 μm in diameter; Sigma-Aldrich, St. Louis, Mo.) were added to PBS to facilitate the removal of adherent E. sakazakii cells from the surfaces of stainless steel and the feeding tubes. PBS (30 ml) containing 3 g of acid-washed glass beads in 50-ml centrifuge tubes was sterilized by autoclaving. All media and the PBS were stored at 4°C and used within 2 days.
Determination of growth curves.
E. sakazakii strains 2855, 3231, 3234, 3295, 3439, and 3396 grown in TSB at 37°C for 24 h were serially diluted in sterile distilled water to make suspensions containing ca. 4 log CFU/ml. Three milliliters of each suspension was added to 300 ml of TSB, IFB, and LJB, and the mixture was shaken at 250 rpm by using a Controlled Environment Incubator Shaker (New Brunswick Scientific Co., Edison, N.J.) for 1 min. The inoculated TSB, IFB, and LJB were incubated at 12 or 25°C for up to 10 days. Populations of E. sakazakii were determined regular intervals up to 10 days. At each sampling time, 0.1 ml was surface plated on TSA by using an Autoplate 4000 Automated Spiral Plater (Spiral Biotech, Norwood, Mass.). Colonies formed on plates incubated at 37°C for 24 h were counted.
Preparation of enteral feeding tubes and stainless steel coupons for attachment and biofilm studies.
Enteral feeding tubes (PVC, 0.2-cm external diameter, Fr 7; Vygon Co., Ltd., Norristown, Pa.) were used. The feeding tubes were cut into 5-cm long pieces with a sterile blade, and the ends of each piece were sealed by melting with flame, followed by applying pressure. The pieces of feeding tubes were immersed in 70% ethanol for 10 min to disinfect the surface, followed by drying in a laminar flow biosafety hood. Each piece of tube was placed in a sterile 15-ml test tube. The outer surface of each piece was 3.14 cm2.
Stainless steel coupons (type 304, 5 by 2 cm, surface area = 20 cm2) with no. 4 finish were also used in attachment and biofilm studies. Coupons were sonicated (model 250D; VWR, Chester, Pa.) in a 15% phosphoric acid solution at 80°C for 20 min, rinsed with distilled water, sonicated in alkali detergent solution (FS Pro-Chlor; Zep, Atlanta, Ga.) at 80°C for 20 min, and rinsed again with distilled water. Washed stainless steel coupons were dried, placed in 50-ml test tubes, and autoclaved.
Preparation of cell suspension for attachment to enteral feeding tubes and stainless steel.
E. sakazakii strains 3231, 3234, 3295, 3439, and 3396 grown in TSB at 37°C for 24 h were serially diluted in sterile distilled water to give ca. 4 log CFU/ml. TSB, IFB, and LJB (500 ml) were inoculated with each cell suspension (5 ml) to give ca. 2 log CFU/ml and mixed thoroughly. The inoculated TSB, IFB, and LJB were incubated at 25°C for 2 days; TSB was incubated at 12°C for 6 days, and IFB and LJB were incubated at 12°C for 8 days.
Attachment of E. sakazakii.
Stationary-phase cultures (25 ml) were deposited in sterile 50-ml test tubes, each containing a sterile stainless coupon; 10 ml of each culture were deposited in a sterile 15-ml test tube containing a sterile piece of feeding tube. Stainless steel coupons and feeding tubes immersed in cultures which had been grown at 12 and 25°C were incubated at 12 and 25°C, respectively, for 4 h to facilitate attachment of the cells, aseptically removed with a sterile forceps, immersed in 400 ml of sterile distilled water (22 ± 2°C), and gently agitated for 15 s. Coupons and tubes were rinsed in 200 ml of sterile distilled water with agitation for 5 s and then deposited in 30 ml of sterile PBS containing 3 g of glass beads in 50-ml centrifuge tubes. The PBS containing a stainless steel coupon or a feeding tube with 3 g of glass beads was vortexed (model Vortex Genie-2; Scientific Industries, Inc., Bohemia, N.Y.) at maximum speed for 1 min. Immediately after vortexing, suspensions were serially diluted in 0.1% peptone water and surface plated (0.1 ml, in duplicate) on TSA to determine the populations of E. sakazakii attached to the surfaces of stainless steel coupons and feeding tubes. Simultaneously, media in which cells had grown and attached to the surfaces of stainless steel coupons and feeding tubes were serially diluted in 0.1% peptone water and surface plated on TSA to determine the number of planktonic cells in the surrounding media.
Biofilm formation.
E. sakazakii strains 3231 and 3439 grown to stationary phase in TSB at 37°C for 24 h were centrifuged (4,000 × g, 15 min, 4°C), and cells were resuspended in PBS (pH 7.4) to give 7 log CFU/ml. Cell suspensions were kept at 4°C for no longer than 30 min to minimize the changes in cell numbers and physiological state before use in experiments. Suspensions (25 ml) of each strain were deposited in sterile 50-ml test tubes containing a sterile stainless coupon; 10 ml of suspension was deposited in a sterile 15-ml test tube containing a sterile piece of feeding tube. After the coupons and tubes were incubated at 4°C for 24 h, they were removed from the test tubes with a sterile forceps, immersed in 400 ml of sterile distilled water (22 ± 2°C) for 15 s, and rinsed in 200 ml of sterile distilled water with gentle agitation for 5 s. The rinsed stainless steel coupons and feeding tubes were deposited, respectively, in 30 or 10 ml of TSB, IFB, or LJB in 50- or 15-ml centrifuge tubes, respectively, and then incubated at 12 or 25°C for up to 10 days. The numbers of E. sakazakii in biofilms formed on coupons and tubes were determined on day 0 (24 h after immersion in PBS cell suspension at 4°C), and after 2, 4, 6, 8, and 10 days at 12°C and 1, 2, 4, 6, 8, and 10 days at 25°C. Coupons and tubes were removed from each medium and rinsed in 400 ml of sterile water (22 ± 2°C) with agitation for 15 s. After another rinse in sterile distilled water (200 ml) for 5 s, the coupons and tubes were placed in 50-ml centrifuge tubes containing 3 g of glass beads and 30 ml of sterile PBS and then vortexed for 1 min. The PBS suspension and each medium in which coupons or tubes had been immersed were serially diluted in 0.1% peptone water, surface plated (0.1 ml, in duplicate) on TSA, and incubated at 37°C. The number of colonies formed on plates within 24 h was counted.
Statistical analysis.
All experiments were performed in triplicate, and two samples representing each combination of test parameters were analyzed at each sampling time. The data were analyzed by using the general linear model of the statistical analysis systems procedure (SAS Institute, Cary, N.C.). The Fisher least-significant-difference test was used to determine whether the attachment and biofilm formation of E. sakazakii on stainless steel coupons and enteral feeding tubes was significantly affected by temperature and the type of media. Significant differences are presented at a 95% confidence level (P ≤ 0.05).
RESULTS AND DISCUSSION
Growth curves of E. sakazakii.
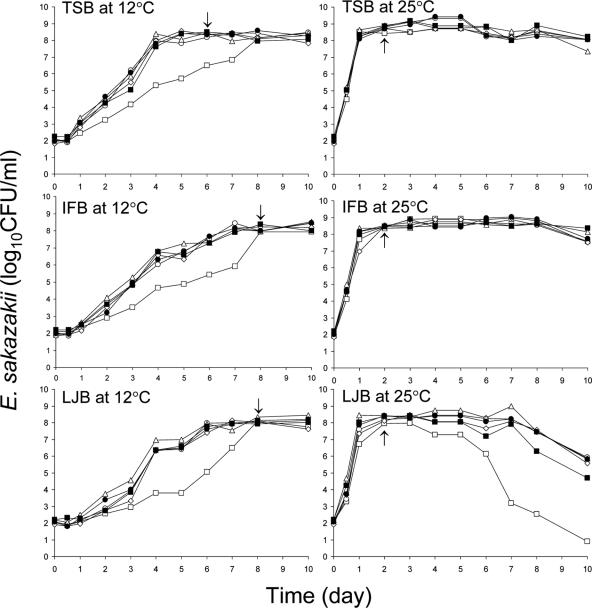
Six strains (2855, 3231, 3234, 3295, 3439, and 3396) of E. sakazakii were examined for growth characteristics in TSB, IFB, or LJB at 12 and 25°C. The growth characteristics varied, depending on the strain, medium, and incubation temperature. Figure 1 shows the growth curves of the six test strains in TSB, IFB, or LJB at 12 and 25°C. All strains except strain 2855 showed very similar growth rates in TSB at 12°C. Strains 3231, 3234, 3295, 3439, and 3396 reached the stationary phase in TSB within 6 days at 12°C, but strain 2855 required about 8 days to reach the stationary phase. Populations of the first five strains increased to 7.63 to 8.38 log CFU/ml within 4 days at 12°C. However, the growth rate of strain 2855 in TSB at 12°C was significantly slower than that of the other five strains. At 25°C, all six strains showed very similar growth patterns in TSB throughout the first 8 days of incubation. Populations of all strains reached 8.09 to 8.64 log CFU/ml within 1 day at 25°C and stationary phase within 1 to 2 days. Growth rates of E. sakazakii in TSB at temperatures ranging from 6 to 49°C were determined by Iversen et al. (23). These authors found that the higher the temperature (<40°C), the faster the rate of growth.
FIG. 1.
Populations of E. sakazakii strains 2855 (□), 3231 (○), 3234 (▵), 3295 (⋄), 3439 (▪), and 3396 (•) grown in TSB, IFB, or LJB at 12 or 25°C for up to 10 days. Arrows indicate early stationary phase of growth for strains 3231, 3234, 3295, and 3396 at 12°C and all test strains at 25°C.
E. sakazakii reached early stationary phase of growth within 7 to 8 days in IFB at 12°C (Fig. 1). With the exception of strain 2855, populations in IFB incubated at 12°C increased to 7.30 to 7.68 log CFU/ml within 6 days. As in TSB, compared to the other five strains, strain 2855 grew markedly slower in IFB. All strains reached the early stationary phase of growth in IFB within 1 to 2 days at 25°C. Others have studied the growth rate of E. sakazakii in infant formulas and cereals. At temperatures between 6 and 30°C, growth rates in TSB were similar to those in infant formula milk (23). In our study, the growth rate of E. sakazakii in IFB at 12°C was slower than that in TSB. Differences in growth rate observed in the two studies are attributed in part to differences in nutrient content of the infant formula. Richards et al. (47) reported that E. sakazakii grew to populations of 7.5 and 4.6 log CFU/ml within 72 h at 21 and 12°C, respectively, in infant rice cereal reconstituted with liquid infant formula initially containing ca. 1.0 log CFU/ml.
The growth curves of E. sakazakii in LJB incubated at 12°C were very similar to those observed in IFB at 12°C (Fig. 1). All test strains reached early stationary phase after 1 to 2 days and after 7 to 8 days of incubation at 12 and 25°C, respectively. E. coli O157:H7 grown in LJB reached the stationary phase of growth within 1 to 2 days at 22°C and 4 to 10 days at 12°C (49). In a previous study (28), we observed that E. sakazakii reached a maximum population (ca. 5 log CFU/ml) within 24 h in unpasteurized lettuce juice at 25°C, began to decrease at 36 h, and was undetectable (<1 log CFU/ml) after 72 h. The LJB used in the present study was sterilized, so a reduction in pH caused by fermentation by microorganisms naturally present in unpasteurized lettuce juice that may otherwise have an effect on viability of E. sakazakii did not play a role. E. sakazakii has been known to survive for >4 days at 12°C when inoculated into unpasteurized lettuce juice at a population of ∼1.5 log CFU/ml (28). Even though all six test strains reached the stationary phase within 2 days at 25°C in IFB and LJB, the death of strain 2855 and the other five strains in LJB began after 3 and 7 days, respectively. Populations of strain 2855 decreased significantly (P ≤ 0.05) from 7.98 log CFU/ml at 3 days to 7.29 log CFU/ml after 4 days, 3.20 log CFU/ml after 7 days, and 0.90 log CFU/ml after 10 days in LJB at 25°C. This strain clearly showed growth and death patterns different from those of the other five strains. Lower amounts and different types of nutrients in LJB, compared to TSB and IFB, may have shortened the stationary phase of growth. A lower buffering capacity of LJB, compared to TSB and IFB, may also have contributed to the rapid initiation of the death phase in LJB. Fermentation of sugars in all test media would result in acid production, with concomitant decreases in pH, particularly if the buffer capacity is minimum as would be the case in LJB. Since the growth rate of strain 2855 in TSB, IFB, or LJB was slower than that of the five other strains in those media, particularly at 12°C, it was not included in subsequent studies on attachment and biofilm formation. The arrows in Fig. 1 indicate the incubation times at which cells were harvested for use in an attachment experiment.
Attachment of E. sakazakii.
Contamination of abiotic surfaces in infant formula preparation and produce processing areas with E. sakazakii resulting from attachment and biofilm formation was simulated in the laboratory. Stainless steel and enteral feeding tubes were used as models, and TSB, IFB, and LJB served as nutrient sources. Although the surface characteristics of cells may vary depending on the medium in which the cells are grown, as well as the phase of growth, cells in early stationary phase were selected for investigation with the goal of minimizing the potential influence of different physiological states on attachment and biofilm formation. The effect of temperature on the attachment of strains 3231, 3234, 3295, 3439, and 3396 grown in TSB, IFB, or LJB at 12 and 25°C to stainless steel and enteral feeding tubes immersed for 4 h at the same respective temperatures in the same respective broths was investigated. Populations of E. sakazakii in early stationary phase that remained in TSB, IFB, or LJB, i.e., planktonic cells, in which tubes were immersed for 4 h at 12 or 25°C, as well as the number of cells which attached to the tubes, are shown in Table 1. Strains 3231 and 3396 attached in significantly higher (P ≤ 0.05) numbers to the feeding tubes immersed in a given broth at 25°C than at 12°C. Strains 3295 and 3439 in TSB and strain 3234 in TSB and IFB attached in significantly higher (P ≤ 0.05) numbers to the feeding tubes at 25°C than at 12°C. Except for strains 3234, 3295, and 3439 at 12°C, significantly higher (P ≤ 0.05) numbers of planktonic cells were recovered from TSB and IFB than from LJB incubated at 12 or 25°C. Higher numbers of planktonic cells in media did not always correlate with higher numbers of cells attached to the tubes. This could be due in part to differences in the initial number of planktonic cells in the three test broths. Except for strain 3234 at 12°C, the cells of test strains attached in significantly higher (P ≤ 0.05) numbers to enteral feeding tubes immersed in IFB than to tubes in LJB incubated at 12 and 25°C. At 25°C, significantly higher (P ≤ 0.05) numbers of all strains attached to the tubes in TSB and IFB than in LJB.
TABLE 1.
Populations of E. sakazakii in suspension and attached to surfaces of enteral feeding tubes immersed in TSB, IFB, or LJB at 12 and 25°C for 4 ha
| Strain | Medium | 12°C
|
25°C
|
||
|---|---|---|---|---|---|
| Attached (log CFU/cm2) | Planktonic (log CFU/ml) | Attached (log CFU/cm2) | Planktonic (log CFU/ml) | ||
| 3231 | TSB | b 5.47 b | b 7.89 a | a 6.09 a | a 8.93 a |
| IFB | b 5.86 a | a 8.17 a | a 6.16 a | a 9.10 a | |
| LJB | b 2.04 c | b 6.09 b | a 4.67 b | a 7.47 b | |
| 3234 | TSB | b 2.88 a | a 8.05 a | a 4.31 a | a 8.99 a |
| IFB | b 2.81 a | b 8.13 a | a 3.55 b | a 8.96 a | |
| LJB | a 2.34 a | a 7.47 a | a 3.05 c | a 8.11 b | |
| 3295 | TSB | b 3.14 b | b 7.86 a | a 5.54 a | a 8.92 a |
| IFB | a 5.17 a | b 7.82 a | a 5.33 a | a 8.73 a | |
| LJB | a 3.60 b | a 7.81 a | a 3.76 b | a 7.52 b | |
| 3439 | TSB | b 3.59 b | b 7.59 a | a 6.03 a | a 8.94 a |
| IFB | a 5.28 a | b 7.75 a | a 5.45 b | a 8.91 a | |
| LJB | a 2.87 b | a 7.20 a | a 3.09 c | a 7.99 b | |
| 3396 | TSB | b 5.06 b | a 8.05 a | a 6.29 a | a 8.79 a |
| IFB | b 5.79 a | b 8.11 a | a 6.28 a | a 9.22 a | |
| LJB | b 3.35 c | b 6.76 b | a 4.81 b | a 8.12 b | |
Within attached cell counts, mean values in the same row that are not preceded by the same letter are significantly different (P ≤ 0.05). Within planktonic cell counts, mean values in the same row that are not preceded by the same letter are significantly different (P ≤ 0.05). Within the same strain, mean values in the same column that are not followed by the same letter are significantly different (P ≤ 0.05).
Compared to cells grown in LJB, cells grown in TSB or IFB may have more ability to attach to the hydrophobic surfaces of enteral feeding tubes. A major difference among the three broths is that TSB and IFB are protein-rich media compared to LJB. The TSB and IFB used in our study both contain casein, and IFB also contains whey protein. Other researchers have determined that nutrients and other components in media affect attachment of microorganisms to surfaces of various materials. Hood and Zottola (20) concluded that composition of growth and conditioning media influence the attachment of Salmonella enterica serovar Typhimurium and Listeria monocytogenes to stainless steel surfaces. These authors observed that S. enterica serovar Typhimurium grown in TSB and L. monocytogenes grown in 1% reconstituted skim milk supplemented with sucrose attach in the highest numbers to stainless steel chips that were conditioned with TSB and 1% reconstituted skim milk, respectively, when TSB, 1% reconstituted skim milk, and 1% reconstituted skim milk with sucrose were used as growth and conditioning media. Casein has been shown to promote attachment of E. coli, Pseudomonas fluorescens, and Aeromonas liquefaciens to glass surfaces (36) and attachment of milk-associated microorganisms to stainless steel surfaces was enhanced in the presence of whey protein (53). The opposite effects were observed by others. Milk and milk protein were reported to inhibit adhesion of L. monocytogenes, serovar Typhimurium, Staphylococcus aureus, Serratia marcescens, and Escherichia coli to abiotic surfaces, including stainless steel or Buna-N rubber (16, 19, 20). These observations were attributed to repulsion between the negative charges of milk protein and bacterial cells (16) and equilibrium between protein in the surrounding medium and proteins that absorb to the contact surfaces (35).
Table 2 shows the populations of E. sakazakii attached to the surface of stainless steel coupons and the numbers of planktonic cells in the media at the end of the 4-h immersion period. Significantly higher (P ≤ 0.05) numbers of strains 3234 and 3439 attached to the surfaces of stainless steel coupons immersed in a given broth at 25°C, compared to 12°C. Strains 3231, 3295, and 3396 also showed the same trend, although in some cases the number of cells that attached at 25°C in a particular broth was not statistically different than the number that attached at 12°C. Attachment of E. sakazakii to the surface of stainless steel was not correlated with the type of medium in which cells had grown or the number of planktonic cells in the surrounding medium. Except for strain 3439, the number of E. sakazakii cells that attached to stainless steel immersed in TSB and IFB was not significantly different than the number that attached to coupons in LJB at 25°C. Under the same attachment conditions, in most cases, higher numbers of E. sakazakii adhered to the feeding tubes than to stainless steel. Stainless steel is moderately hydrophilic, whereas PVC (the enteral feeding tube) is highly hydrophobic. Most bacteria adhere more readily to hydrophobic surfaces (46).
TABLE 2.
Populations of E. sakazakii in suspension and attached to surfaces of stainless steel coupons immersed in TSB, IFB, or LJB at 12 and 25°C for 4 ha
| Strain | Medium | 12°C
|
25°C
|
||
|---|---|---|---|---|---|
| Attached (log CFU/cm2) | Planktonic (log CFU/ml) | Attached (log CFU/cm2) | Planktonic (log CFU/ml) | ||
| 3231 | TSB | b 3.55 b | a 7.98 b | a 4.50 a | a 8.83 a |
| IFB | b 3.41 b | a 8.57 a | a 4.28 a | a 9.14 a | |
| LJB | a 4.19 a | a 7.65 b | a 4.45 a | a 7.69 b | |
| 3234 | TSB | b 3.46 a | a 8.24 a | a 4.17 a | a 8.85 b |
| IFB | b 3.01 b | a 7.96 a | a 4.02 a | a 9.25 a | |
| LJB | b 2.30 c | a 7.55 a | a 3.71 a | a 7.65 c | |
| 3295 | TSB | b 2.92 b | a 7.30 a | a 4.53 a | a 8.39 a |
| IFB | a 4.06 a | b 7.54 a | a 4.10 a | a 8.87 a | |
| LJB | b 3.31 b | a 7.41 a | a 4.81 a | a 7.22 b | |
| 3439 | TSB | b 3.64 a | a 8.01 a | a 4.62 a | a 9.03 a |
| IFB | b 3.60 a | a 7.82 a | a 4.31 a | a 8.11 ab | |
| LJB | b 3.01 b | a 7.36 a | a 3.95 b | a 7.54 b | |
| 3396 | TSB | b 3.54 a | a 7.49 a | a 5.11 a | a 8.56 a |
| IFB | a 4.07 a | b 7.79 a | a 4.78 a | a 8.74 a | |
| LJB | a 4.02 a | a 7.74 a | a 4.36 a | a 7.64 b | |
Within attached cell counts, mean values in the same row that are not preceded by the same letter are significantly different (P ≤ 0.05). Within planktonic cell counts, mean values in the same row that are not preceded by the same letter are significantly different (P ≤ 0.05). Within the same strain, mean values in the same column that are not followed by the same letter are significantly different (P ≤ 0.05).
The influences of temperature on the attachment of other food-borne pathogens to biotic and abiotic surfaces have been investigated by others. Our observations are in agreement with a study by Gorski et al. (14), who reported that temperature plays a role in the attachment of L. monocytogenes to radish tissue. In that study, L. monocytogenes cells were found to attach in higher numbers to the radish tissues at 20°C than at 10°C after ≥1 h of exposure. Higher numbers of E. coli O157:H7 attached to lettuce leaves at 22°C than at 4 or 10°C (54). Factors affecting attachment at different temperatures, however, may vary, depending on the nature of surfaces to which the cells are exposed. Pompermayer and Gaylarde (43), for example, showed that higher numbers of S. aureus attached to polypropylene at 12°C than at 30°C after ≥4 h of contact time. They also observed that the numbers of E. coli attached to polypropylene within 4 h of exposure at 12°C and 30°C were not significantly different. The adhesion of Salmonella enterica serovar Montevideo to tomatoes and tomatillos was not influenced by temperature alone (12, 22, or 30°C) but also by combinations of temperature and relative humidity (21). E. sakazakii grown under different conditions in our study could have had different surface charges and hydrophobicity, which are known to affect the ability of bacterial cells to adhere to surfaces at various temperatures (10, 56).
Biofilm formation by E. sakazakii.
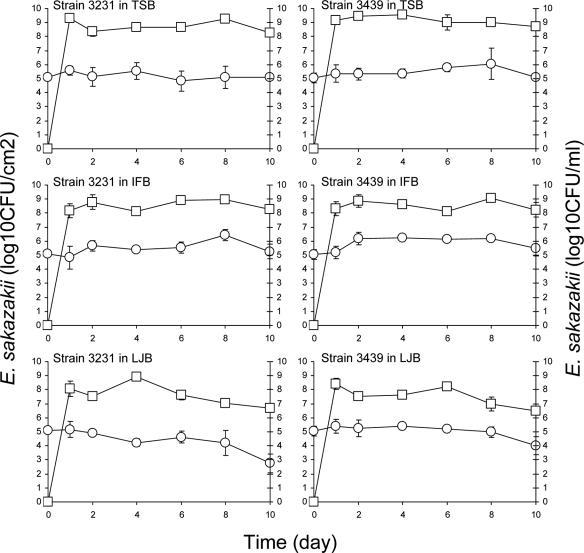
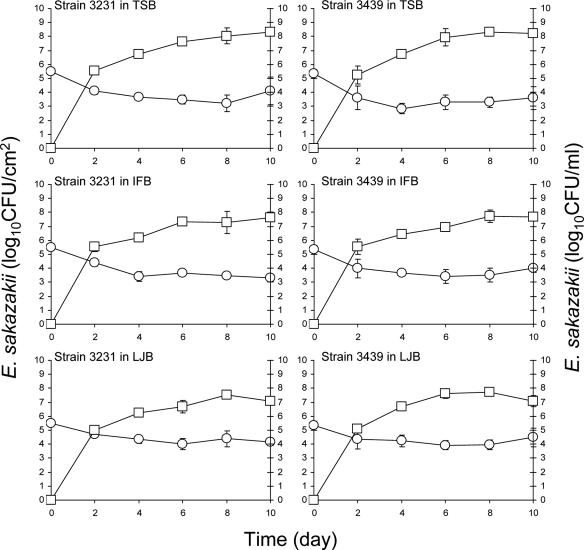
Stainless steel coupons and enteral feeding tubes were immersed in cell suspensions in PBS (ca. 7 log CFU/ml) at 4°C for 24 h to facilitate attachment, followed by rinsing, transfer into TSB, IFB, or LJB, and incubation at 12 or 25°C for up to 10 days. Figure 2 shows the number of E. sakazakii cells (strains 3231 and 3439) retrieved from biofilms that formed on feeding tubes. The number of E. sakazakii cells initially attached to the surface of the feeding tubes did not change significantly (P ≤ 0.05) during immersion in TSB at 25°C. Some of the cells would be expected to naturally detach from the surface due to the changes in physiochemical properties (49), starvation (44), and death but, on balance, the number remained the same throughout the 10-day period. Equilibrium was apparently reached between the number of cells that detached from the tube surface, newly attached cells originating from TSB, and newly formed cells originating from cells attached to or in biofilms on the tube surface. Populations of planktonic cells of strains 3231 and 3439 in TSB increased to ≥9.15 log CFU/ml within 1 day, which was followed by slight decreases during after 9 days. E. sakazakii initially attached to the feeding tubes grew when the tubes were immersed in IFB at 25°C. The populations of strains 3231 and 3439 increased significantly (P ≤ 0.05) from initial populations of 5.12 and 5.03 CFU/cm2, respectively, to 6.43 and 6.20 log CFU/cm2 on the feeding tube surface at 8 days. Planktonic E. sakazakii in IFB in which tubes were immersed reached the stationary phase of growth within 2 days. Populations on tubes immersed in LJB at 25°C did not change significantly for the first 8 days but decreased between 8 and 10 days. Planktonic E. sakazakii in LJB reached the stationary phase of growth within 2 days. The death phase for strains 3231 and 3439 began at 4 and 6 days, respectively.
FIG. 2.
Populations of E. sakazakii strains 3231 and 3396 attached to the surface or in biofilms formed on the surface of enteral feeding tubes (log CFU/cm2, ○) immersed in TSB, IFB, or LJB or in broths (log CFU/ml, □) in which tubes were immersed and incubated at 25°C for up to 10 days. Bars indicate the standard deviations.
Populations of E. sakazakii strains 3231 and 3439 recovered from the surface of stainless steel coupons immersed in TSB, IFB, and LJB at 25°C are shown in Fig. 3. The number of E. sakazakii strains 3231 and 3439 initially attached to the stainless steel did not change significantly when coupons were immersed in TSB. Initial populations (5.33 to 5.51 log CFU/cm2) on coupons immersed in IFB increased significantly (P ≤ 0.05) to 6.69 to 6.89 log CFU/cm2 within 10 days. The infant formula used to prepare IFB contains nonfat milk, corn syrup, lactose, soy oil, whey protein concentrate, and other nutrients, thus providing protein, fat, carbohydrate, vitamins, and minerals as nutrient sources. As noted above, milk or milk proteins have been shown to either enhance or inhibit bacterial attachment to abiotic surfaces. In our study, E. sakazakii attached to surfaces of stainless steel in a PBS suspension, followed by immersion in IFB. The IFB may provide the nutrients necessary for E. sakazakii cells preattached to the stainless steel or feeding tubes to survive and grow. This observation is in agreement with the study by Helke and Wong (17), who reported that L. monocytogenes cells preattached to stainless steel grew in the presence of milk at 25°C. We observed that as incubation time progressed at 25°C, the texture of IFB became markedly viscous. The high viscosity of IFB may facilitate harborage of E. sakazakii cells. Biofilm formation by E. sakazakii on latex, silicon, and stainless steel immersed in infant formula milk at 37°C has been reported (23). Lehner et al. (32) observed that biofilms were formed by 59% of E. sakazakii test strains in PVC microtiter wells and extracellular polysaccharide was produced by 42.8% of the test strains. Extracellular polysaccharide is known to be involved in bacterial adhesion and biofilm formation (6, 7, 11). In the temperature rage of 0 to 30°C, the highest amount of polysaccharide synthesized by E. sakazakii was at 27°C (50).
FIG. 3.
Populations of E. sakazakii strains 3231 and 3396 attached to the surface or in biofilms formed on the surface of stainless steel coupons (log CFU/cm2, ○) immersed in TSB, IFB, or LJB or in broths (log CFU/ml, □) in which coupons were immersed and incubated at 25°C for up to 10 days. Bars indicate the standard deviations.
Populations of E. sakazakii initially attached to stainless steel remained constant for 2 to 4 days when immersed in LJB and then significantly decreased (P ≤ 0.05) by ≥2.38 log CFU/cm2 after 10 days (Fig. 3). Planktonic E. sakazakii in the LJB reached 7.82 to 8.15 log CFU/ml within 1 day, followed by slow decreases during the remainder of the 10-day incubation period. A similar phenomenon was reported by Ryu et al. (49), who showed that E. coli O157:H7 attached to stainless steel coupons did not form biofilm when immersed in LJB. The number of E. sakazakii and E. coli O157:H7 cells that detached from the surface of stainless steel is apparently higher than the number of newly attached cells and preattached cells that grow. A lack of nutrients in LJB, compared to TSB and IFB, as well as a reduction in pH during incubation, may also contribute to significant decreases in the surface populations.
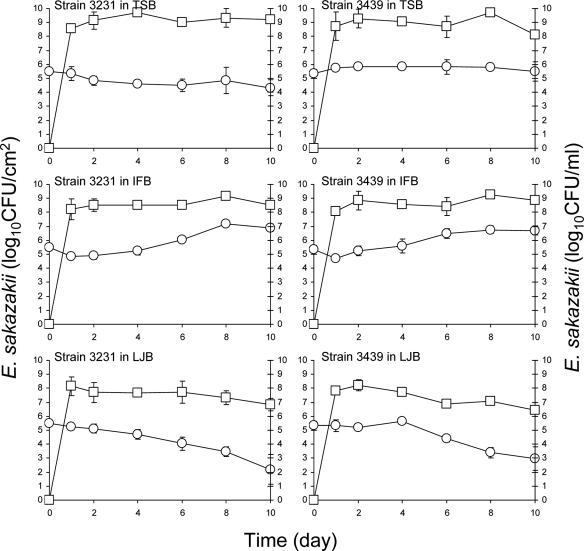
Figure 4 shows the number of E. sakazakii strains 3231 and 3439 detected on the surface of enteral feeding tubes and its growth in TSB, IFB, and LJB in which tubes were immersed at 12°C. None of the strains produced biofilms on tubes immersed in TSB. Unlike the behavior of cells in TSB at 25°C, the number of adherent cells gradually decreased at 12°C. Since growth of E. sakazakii is very slow at 12°C, biofilm formation in TSB was also restricted. Decreases in the populations of the adherent cells were observed within 2 days. Even though E. sakazakii reached a stationary growth phase in TSB in which tubes were immersed within 8 to 10 days, the number of the cells remaining attached to the surfaces of tubes simultaneously decreased. This may be due in part to the presence of fewer planktonic cells at 12°C than at 25°C; to a slower rate of growth of cells preattached to the surfaces at 12°C; and to different physiological states of the cells in media, which has been reported to affect the cell surface characteristics of E. coli (57). E. sakazakii, initially attached at 5.03 to 5.12 log CFU/cm2 of the tube surface, decreased significantly (P ≤ 0.05) on tubes immersed in IFB at 12°C. This is in contrast to its behavior at 25°C (Fig. 2) and indicates that temperature plays an important role in biofilm formation by E. sakazakii in IFB. E. sakazakii reached the stationary phase of growth in IFB surrounding the feeding tubes within 8 to 10 days (Fig. 4). Reductions in the numbers of adherent cells on the surface of feeding tubes immersed in LJB at 12°C were 1.23 to 1.47 log CFU/cm2, which are not substantially different than the 1.05 to 2.37 log CFU/cm2 reduction at 25°C (Fig. 2).
FIG. 4.
Populations of E. sakazakii strains 3231 and 3396 attached to the surface or in biofilms formed on the surface of enteral feeding tubes (log CFU/cm2, ○) immersed in TSB, IFB, or LJB or in broths (log CFU/ml, □) in which tubes were immersed and incubated at 12°C for up to 10 days. Bars indicate the standard deviations.
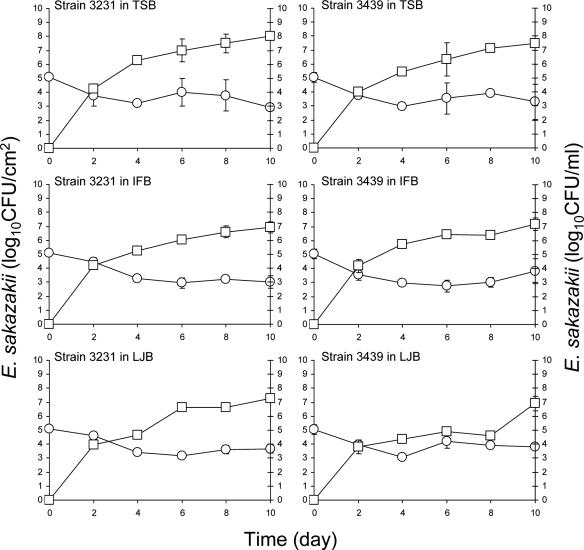
Figure 5 shows the number of E. sakazakii strains 3231 and 3439 on stainless steel coupons immersed in TSB, IFB, or LJB at 12°C and in the immersion broths. As with feeding tubes incubated at 12°C, none of the strains formed biofilms on stainless steel in TSB. Strains 3231 and 3439 attached to stainless steel coupons in PBS cell suspension (ca. 7 log CFU/ml) during incubation at 4°C for 24 h at populations of 5.51 and 5.33 log CFU/cm2. Immersion of these coupons in TSB resulted in a reduction of 1.4 log CFU/cm2 for strain 3231 and 1.7 log CFU/cm2 for strain 3439 during incubation at 12°C for 10 days. E. sakazakii reached the stationary phase of growth in TSB at 25°C within 1 to 2 days (Fig. 3) but required 6 to 8 days when incubated at 12°C (Fig. 5). Numbers of the cells in stationary phase at 12°C reached ≥8.0 log CFU/ml of TSB after 8 days. Unlike the significant growth of E. sakazakii observed on stainless steel in IFB at 25°C, the initial numbers of adherent cells decreased significantly (P ≤ 0.05) upon reaching 1.33 to 2.20 log CFU/cm2 after 10 days at 12°C. E. sakazakii did not form biofilm on stainless steel immersed in LJB at 12°C, as was the case at 25°C. However, a slower reduction occurred in the number of cells adhering to stainless steel immersed in LJB at 12°C than at 25°C. Populations of strains 3231 and 3439 attached to stainless steel in LJB decreased by 3.32 and 2.38 log CFU/cm2, respectively, at 25°C (Fig. 3) but only 1.37 and 0.84 log CFU/cm2, respectively, at 12°C (Fig. 5). The larger decreases in populations at 25°C than at 12°C are attributed in part to the death of adherent cells on stainless steel immersed in LJB at 25°C. Like its behavior in TSB and IFB at 12°C, E. sakazakii also reached the stationary phase of growth in LJB after 8 to 10 days. E. sakazakii behaved similarly in TSB, IFB, and LJB at 12°C. Differences in the composition of three media did not have an influence on the biofilm formation of E. sakazakii at 12°C. The results suggest that nutrient depletion did not cause great decreases in the populations of cells attached to feeding tubes or stainless steel coupons at 12°C. It is not the surrounding medium or incubation temperature alone but rather a combination of medium, temperature, or other factors that influence the biofilm formation of E. sakazakii.
FIG. 5.
Populations of E. sakazakii strains 3231 and 3396 attached to the surface or in biofilms formed on the surface of stainless steel coupons (log CFU/cm2, ○) immersed in TSB, IFB, or LJB or in broths (log CFU/ml, □) in which coupons were immersed and incubated at 12°C for up to 10 days. Bars indicate the standard deviations.
In summary, E. sakazakii attached better to the surfaces of enteral feeding tubes and stainless steel in TSB, IFB, or LJB at 25°C than at 12°C. E. sakazakii, preattached to stainless steel and enteral feeding tubes and immersed in IFB, formed biofilms at 25°C. The importance of preventing the contamination of contact surfaces in areas where powdered infant formulas are reconstituted or fed to infants is reinforced by observations that E. sakazakii can attach to and form biofilms on these surfaces. The results emphasize the importance of temperature control in the infant formula and produce processing industries to inhibit attachment and biofilm formation by E. sakazakii. Further studies are needed to determine the resistance of E. sakazakii in biofilm to various sanitizers and disinfectants used in these industries and in formula reconstitution and feeding areas.
REFERENCES
- 1.Bar-Oz, B., A. Preminger, O. Peleg, C. Block, and I. Arad. 2001. Enterobacter sakazakii infection in the newborn. Acta Pediatr. 90:356-358. [PubMed] [Google Scholar]
- 2.Beuchat, L. R. 2002. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 4:413-423. [DOI] [PubMed] [Google Scholar]
- 3.Biering, G., S. Karlsson, N. V. C. Clark, K. E. Jonsdottir, P. Ludvigsson, and O. Steingrimsson. 1989. Three cases of neonatal meningitis caused by Enterobacter sakazakii in powdered milk. J. Clin. Microbiol. 27:2054-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burdette, J. H., and C. Santos. 2000. Enterobacter sakazakii brain abscess in the neonate: the importance of neuroradiologic imaging. Pediatr. Radiol. 30:33-34. [DOI] [PubMed] [Google Scholar]
- 5.Cruz, A. C., E. Fernandez, E. Salinas, P. Ramirez, C. Montiel, and C. A. Eslava. 2004. Characterization of Enterobacter sakazakii isolated from different sources. Abstr. 104th Gen. Meet. Am. Soc. Microbiol., abstr. Q-051.
- 6.Danese, P. N., L. A. Pratt, and R. Kolter. 2000. Exopolysaccharide production is required for the development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies, D. G., and G. G. Geesey. 1995. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl. Environ. Microbiol. 61:860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelson-Mammel, S. G., and R. L. Buchanan. Thermal inactivation of Enterobacter sakazakii in rehydrated infant formula. J. Food Prot. 67:60-63. [DOI] [PubMed]
- 9.Emery, C. L., and L. A. Weymouth. 1997. Detection and clinical significance of extended-spectrum beta-lactamases in a tertiary-care medical center. J. Clin. Microbiol. 35:2061-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher, M., and G. I. Loeb. 1979. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl. Environ. Microbiol. 37:67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank, J. F. 2001. Microbial attachment to food and food contact surfaces. Adv. Food. Nutr. Res. 43:319-370. [DOI] [PubMed] [Google Scholar]
- 12.Frank, J. F., J. Ehlers, and L. Wicker. 2003. Removal of Listeria monocytogenes and poultry soil-containing biofilms using chemical cleaning and sanitizing agents under static conditions. Food Prot. Trends 23:654-663. [Google Scholar]
- 13.Gallagher, P. G., and W. S. Ball. 1991. Cerebral infarction due to CNS infection with Enterobacter sakazakii. Pediatr. Radiol. 21:135-136. [DOI] [PubMed] [Google Scholar]
- 14.Gorski, L., J. D. Palumbo, and R. E. Mandrell. 2003. Attachment of Listeria monocytogenes to radish tissue is dependent upon temperature and flagellar motility. Appl. Environ. Microbiol. 69:258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawkins, R. E., C. R. Lissner, and J. P. Sanford. 1991. Enterobacter sakazakii bacteremia in an adult. South. Med. J. 84:793-795. [DOI] [PubMed] [Google Scholar]
- 16.Helke, D. M., E. B. Somers, and A. C. L. Wong. 1993. Attachment of Listeria monocytogenes and Salmonella typhimurium to stainless steel and Buna-N in the presence of milk and individual milk components. J. Food Prot. 56:479-484. [DOI] [PubMed] [Google Scholar]
- 17.Helke, D. M., and A. C. L. Wong. 1994. Survival and growth characteristics of Listeria monocytogenes and Salmonella typhimurium on stainless steel and buna-n rubber. J. Food Prot. 57:963-968. [DOI] [PubMed] [Google Scholar]
- 18.Himelright, I., E. Harris, V. Lorch, and M. Anderson. 2002. Enterobacter sakazakii infections associated with the use of powdered infant formula-Tennessee, 2001. JAMA 287:2204-2205. [PubMed] [Google Scholar]
- 19.Hood, S. K., and E. A. Zottola. 1997. Adherence of stainless steel by foodborne microorganisms during growth in model food systems. Int. J. Food Microbiol. 37:145-153. [DOI] [PubMed] [Google Scholar]
- 20.Hood, S. K., and E. A. Zottola. 1997. Growth media and surface conditioning influence the adherence of Pseudomonas fragi, Salmonella typhimurium, and Listeria monocytogenes cells to stainless steel. J. Food Prot. 60:1034-1037. [DOI] [PubMed] [Google Scholar]
- 21.Iturriaga, M. H., E. F. Escartín, L. R. Beuchat, and R. Martinez-Peniche. 2003. Effect of inoculum size, relative humidity, storage temperature, and ripening stage on the attachment of Salmonella Montevideo to tomatoes and tomatillos. J. Food Prot. 66:1756-1761. [DOI] [PubMed] [Google Scholar]
- 22.Iversen, C., and S. J. Forsythe. 2003. Risk profile of Enterobacter sakazakii, an emergent pathogen associated with infant milk formula. Trends Food Sci. Technol. 14:443-454. [Google Scholar]
- 23.Iversen, C., M. Lane, and S. J. Forsythe. 2004. The growth profile, thermotolerance and biofilm formation of Enterobacter sakazakii grown in infant formula milk. Lett. Appl. Microbiol. 38:378-382. [DOI] [PubMed] [Google Scholar]
- 24.Iversen, C., M. Waddington, S. L. W. On, and S. Forsythe. 2004. Identification and phylogeny of Enterobacter sakazakii relative to Enterobacter and Citrobacter species. J. Clin. Microbiol. 42:5368-5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez, E. B., and C. R. Gimenez. 1982. Septic shock due to Enterobacter sakazakii. Clin. Microbiol. Newslett. 4:30. [Google Scholar]
- 26.Jung, M.-K., and Park, J.-H. 2006. Prevalence and thermal stability of Enterobacter sakazakii from unprocessed ready-to-eat agricultural products and powdered infant formulas. Food Sci. Biotechnol. 15:152-157. [Google Scholar]
- 27.Kandhai, C. M., M. W. Reij, L. G. M. Gorris, O. Guilaume-Gentil, and M. van Schothorst. 2004. Occurrence of Enterobacter sakazakii in food production environments and households. Lancet 363:39-40. [DOI] [PubMed] [Google Scholar]
- 28.Kim, H., and L. R. Beuchat. 2005. Survival and growth of Enterobacter sakazakii on fresh-cut fruits and vegetables and in unpasteurized juice as affected by storage temperature. J. Food Prot. 68:2541-2552. [DOI] [PubMed] [Google Scholar]
- 29.Kumar, C. G., and S. K. Anand. 1998. Significance of microbial biofilms in food industry: a review. Int. J. Food Microbiol. 42:9-27. [DOI] [PubMed] [Google Scholar]
- 30.Lai, K. K. 2001. Enterobacter sakazakii infections among neonates; infants, children, and adults: case reports and a review of the literature. Medicine 80:113-122. [DOI] [PubMed] [Google Scholar]
- 31.Leclercq, A., C. Wanegue, and P. Baylac. 2002. Comparison of fecal coliform agar and violet red bile lactose agar for fecal coliform enumeration in foods. Appl. Environ. Microbiol. 68:1631-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehner, A., K. Riedel, L. Eberl, P. Breeuwer, B. Diep, and R. Stephan. 2005. Biofilm formation, extracellular polysaccharide production, and cell-to-cell signaling in various Enterobacter sakazakii strains: aspects promoting environmental persistence. J. Food Prot. 68:2287-2294. [DOI] [PubMed] [Google Scholar]
- 33.Lehner, A., T. Tasara, and R. Stephan. 2004. 16S rRNA gene based analysis of Enterobacter sakazakii strains from different sources and development of a PCR assay identification. BMC Microbiol. 4:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall, K. C. 1992. Biofilms: an overview of bacterial adhesion, activity, and control at surfaces. ASM News 58:202-207. [Google Scholar]
- 35.McGuire, J. 1989. A predictive model for food particle interactions with contact surfaces. J. Food Sci. 54:22-29. [Google Scholar]
- 36.Meadows, P. S. 1971. The attachment of bacteria to solid surfaces. Arch. Mikrobiol. 75:374-381. [DOI] [PubMed] [Google Scholar]
- 37.Muytjens, H. L., and W. H. Roelofs, and G. H. J. Jasper. 1988. Quality of powdered substitutes for breast milk with regard to members of the family Enterobacteriaceae. J. Clin. Microbiol. 26:743-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muytjens, H. L., H. C. Zanen, H. J. Sonderkamp, L. A. Kolee, I. K. Wachsmuth, and J. J. Farmer. 1983. Analysis of eight cases of neonatal meningitis and sepsis due to Enterobacter sakazakii. J. Clin. Microbiol. 18:115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nazarowec-White, M., and J. M. Farber. 1997. Incidence, survival, and growth of Enterobacter sakazakii in infant formula. J. Food Prot. 60:226-230. [DOI] [PubMed] [Google Scholar]
- 40.Noriega, F. R., K. L. Kotloff, M. A. Martin, and R. S. Schwalbe. 1990. Nosocomial bacteremia caused by Enterobacter sakazakii and Leuconostoc mesenteroides resulting from extrinsic contamination of infant formula. Pediatr. Infect. Dis. 9:447-449. [PubMed] [Google Scholar]
- 41.Norwood, D. E., and A. Gilmour. 2000. The growth and resistance to sodium hypochlorite of Listeria monocytogenes in a steady-state multispecies biofilm. J. Appl. Microbiol. 88:512-520. [DOI] [PubMed] [Google Scholar]
- 42.Oie, S., and A. Kamiya. 2001. Comparison of microbial contamination of enteral feeding solution between repeated use of administration sets after washing with water and after washing followed by disinfection. J. Hosp. Infect. 48:304-307. [DOI] [PubMed] [Google Scholar]
- 43.Pompermayer, D. M. C., and C. C. Gaylarde. 2000. The influence of temperature on the adhesion of mixed cultures of Staphylococcus aureus and Escherichia coli to polypropylene. Food Microbiol. 17:361-365. [Google Scholar]
- 44.Poulsen, L. V. 1999. Microbial biofilm in food processing. Lebensm. Wiss. Technol. 32:321-326. [Google Scholar]
- 45.Pribyl, C., R. Salzer, J. Beskin, R. J. Haddad, B. Pollock, R. Beville, B. Holmes, and W. J. Mogabgab. 1985. Aztreonam in the treatment of serious orthopedic infections. Am. J. Med. 78:51-56. [DOI] [PubMed] [Google Scholar]
- 46.Pringle, J. H., and M. Fletcher. 1983. Influence of substratum wettability on attachment of fresh water bacteria to solid surfaces. Appl. Environ. Microbiol. 45:811-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richards, G. M., J. B. Gurtler, and L. R. Beuchat. 2005. Survival and growth of Enterobacter sakazakii in infant rice cereal reconstituted with water, milk, liquid infant formula, or apple juice. J. Appl. Microbiol. 99:844-850. [DOI] [PubMed] [Google Scholar]
- 48.Ryu, J.-H., and L. R. Beuchat. 2005. Biofilm formation by Escherichia coli O157:H7 on stainless steel: effect of exopolysaccharide and Curli production on its resistance to chlorine. Appl. Environ. Microbiol. 71:247-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryu, J.-H., H. Kim, and L. R. Beuchat. 2004. Attachment and biofilm formation by Escherichia coli O157:H7 on stainless steel as influenced by exopolysaccharide production, nutrient availability, and temperature. J. Food Prot. 67:2123-2131. [DOI] [PubMed] [Google Scholar]
- 50.Scheepe-Leberkühne, M., and F. Wagner. 1986. Optimization and preliminary characterization of an ecopolysaccharide synthesized by Enterobacter sakazakii. Biotechnol. Lett. 8:695-700. [Google Scholar]
- 51.Simmons, B. P., M. S. Gelfand, M. Haas, L. Metts, and J. Ferguson. 1989. Enterobacter sakazakii infections in neonates associated with intrinsic contamination of a powdered infant formula. Infect. Control Hosp. Epidemiol. 10:398-401. [DOI] [PubMed] [Google Scholar]
- 52.Soriano, J. M., H. Rico, J. C. Moltó, and J. Mañes. 2001. Incidence of microbial flora in lettuce, meat and Spanish potato omelette from restaurants. Food Microbiol. 18:159-163. [Google Scholar]
- 53.Speers, J. G. S., and A. Gilmour. 1985. The influence of milk and milk components on the attachment of bacteria to farm dairy equipment surfaces. J. Appl. Bacteriol. 59:325-332. [DOI] [PubMed] [Google Scholar]
- 54.Takeuchi, K., A. N. Hassan, and J. F. Frank. 2001. Penetration of Escherichia coli O157:H7 into lettuce as influenced by modified atmosphere and temperature. J. Food Prot. 64:1820-1823. [DOI] [PubMed] [Google Scholar]
- 55.Van Acker, V., F. de Smet, G. Muyldermans, A. Bougatef, A. Naessens, and S. Lauwers. 2001. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J. Clin. Microbiol. 39:293-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Loosdrecht, M. C., J. Lyklema, W. Norde, G. Schraa, and A. J. Zehnder. 1987. The role of bacterial cell wall hydrophobicity in adhesion. Appl. Environ. Microbiol. 53:1893-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker, S. L., J. E. Hill, J. A. Redman, and M. Elimelech. 2005. Influence of growth phase on adhesion kinetics of Escherichia coli D21g. Appl. Environ. Microbiol. 71:3093-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams, T. L., S. R. Monday, S. Edelso-Mammel, R. Buchanan, and S. M. Musser. 2005. A top-down proteomics approach for differentiating thermal resistant strains of Enterobacter sakazakii. Proteomics 5:4161-4169. [DOI] [PubMed] [Google Scholar]