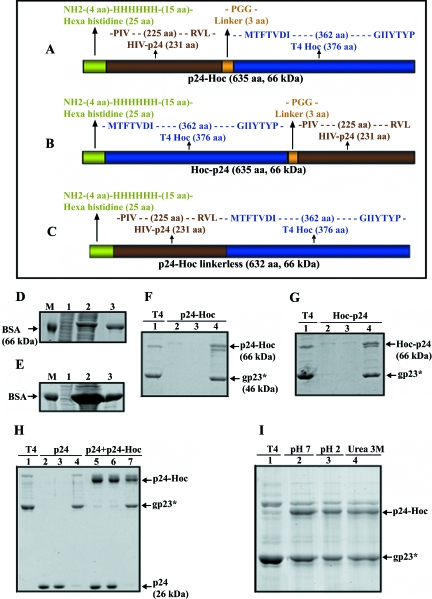
FIG. 2.
In vitro display of HIV p24 on phage T4. p24 was fused in frame to the N terminus (A) or C terminus (B) of Hoc or to the N terminus of Hoc without the Pro-Gly-Gly linker (C) (see Materials and Methods). (D and E) Expression and purification of p24-Hoc (D) and Hoc-p24 (E). Lanes: 1 and 2, E. coli samples analyzed by SDS-10% PAGE before (0 h) or after (3 h) IPTG induction, respectively; 3, purified protein after Ni-affinity chromatography; M, BSA (66 kDa) used as a molecular mass standard. (F and G) In vitro assembly of p24-Hoc (F) and Hoc-p24 (G) on hoc−soc− phage (see Materials and Methods). Lanes: 1, control hoc−soc− phage; 2, starting p24-Hoc or Hoc-p24; 3, supernatant containing the unbound protein; 4, phage pellet containing the displayed protein. The samples were electrophoresed by SDS-4 to 20% PAGE and stained with Coomassie blue. (On a 4 to 20% gradient SDS-PAGE gel, p24-Hoc migrates slightly slower than gp18 [see, for example, panels F and G], whereas on an SDS-10% PAGE gel, it migrates faster [see, for example, Fig. 3A]). This anomalous behavior of Hoc is linked to the C-terminal domain of Hoc, although the reasons are unknown (unpublished results).(H) Specificity of in vitro binding. hoc−soc− particles (lane 1) were incubated with either p24 (lanes 2 to 4) or a mixture of p24 and p24-Hoc (lanes 5 to 7) under standard assembly conditions, and the unbound and bound fractions were analyzed. A 25:1 ratio of p24 (lane 2) or a mixture of p24 and p24-Hoc (lane 5) to capsid binding sites was used. Lanes: 2 and 5, starting p24, or p24 and p24-Hoc mixture; 3 and 6, supernatants containing the unbound proteins; 4 and 7, phage pellet containing the displayed protein. (I) Stability of displayed p24-T4 phage. The displayed p24-T4 particles were treated with control pH 7 buffer (lane 2), pH 2 buffer (lane 3), or 3 M urea (lane 4). Lane 1 (T4) represents control hoc−soc− phage. The samples in panels H and I were electrophoresed on a SDS-10% PAGE gel and stained with Coomassie blue.