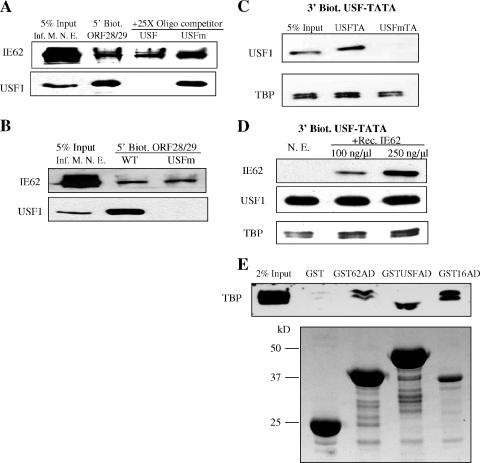
FIG. 7.
Analysis of the binding of IE62, USF, and TBP to promoters. (A) The effect of USF binding on IE62 recruitment to the ORF28/29 regulatory element. The 210-bp 5′-biotinylated ORF28/29 regulatory element (5′ Biot. ORF28/29) was conjugated to magnetic beads and incubated with nuclear extracts derived from VZV-infected MeWo cells (Inf. M. N. E.). Oligomers (22 bp) containing the wild-type or mutant USF binding site (USF or USFm, respectively) were used as competitors in the incubation. The presence or absence of IE62 and USF1 in eluates was determined by immunoblotting. (B) Immunoblot analysis of binding of IE62 present in infected cell nuclear extracts to the ORF28/29 regulatory element containing either the wild-type or mutant USF binding site (5′ Biot. ORF28/29 WT and USFm, respectively). (C) USF1 and TBP binding to the model USF-TATA promoter. Bead-immobilized 132-bp 3′-biotinylated USF-TATA promoter sequences containing the mutant or wild-type USF binding site (USFm or USF, respectively) were incubated with 250 μg nuclear extracts of uninfected MeWo cells. The levels of USF1 and TBP were determined by immunoblotting. (D) Effect of IE62 on TBP binding to the wild-type model promoter. Bead-immobilized 3′-biotinylated USF-TATA promoter was incubated with nuclear extracts of uninfected MeWo cells (N. E.) with or without preincubation with purified recombinant IE62 (Rec. IE62) present in increasing amounts. The presence of IE62, USF1, and TBP stably associated with the promoter was determined by immunoblotting following elution. (E) Interaction of the IE62, USF1, and VP16 ADs present as GST fusions in protein pull-down assays. The activation domain fusions were expressed in E. coli using the pGST-IE62AD, pGST-USF1AD, and pGST-VP16AD plasmids. The upper panel is an immunoblot showing the levels of TBP/TFIID detected in eluates from glutathione beads. The lower panel is a Coomassie blue-stained gel showing the levels of the fusion proteins and GST which coeluted from the beads. The difference in the position of the TBP band between the GSTUSFAD lane and the other lanes is due to distortion resulting from the high level of the recombinant GSTUSFAD fusion, which migrates with a mobility very similar to that of TBP.