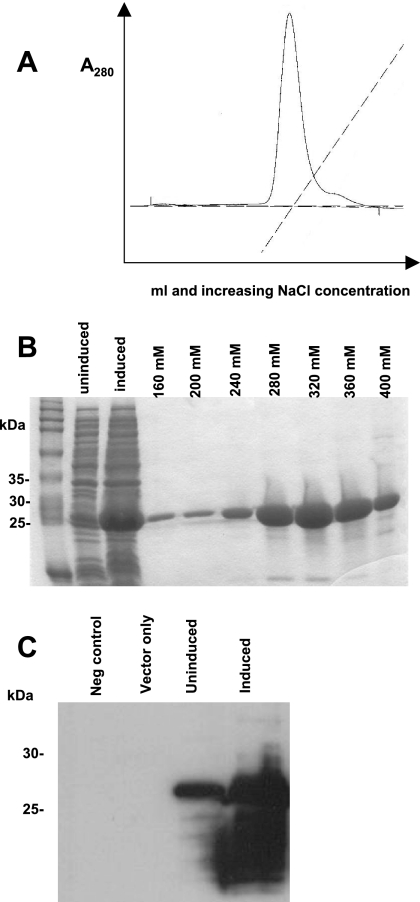
FIG. 4.
Expression and purification of recombinant WT SIV capsid proteins. (A) Proteins were purified from E. coli by anion-exchange chromatography and eluted with a 0 to 1 M NaCl gradient as indicated by the dashed line. (B) Coomassie blue stain of the SDS-PAGE analysis of the following: lane 1, total cellular BL21(DE3)-RIL E. coli proteins prior to induced expression of the capsid protein; lane 2, total cellular bacterial proteins following induction; lanes 3 to 9, SIV CA fractions that were eluted, pooled, and concentrated. The 27-kDa SIV WT CA was optimally eluted at 280 to 320 mM NaCl. The protein preparations were >90% homogeneous. (C) The identity of the proteins was verified by Western blotting using a mouse monoclonal antibody, 55-2F12, directed against SIV CA. The SIV CA is 27 kDa. The positions of migration and molecular mass in kDa are indicated on the left.