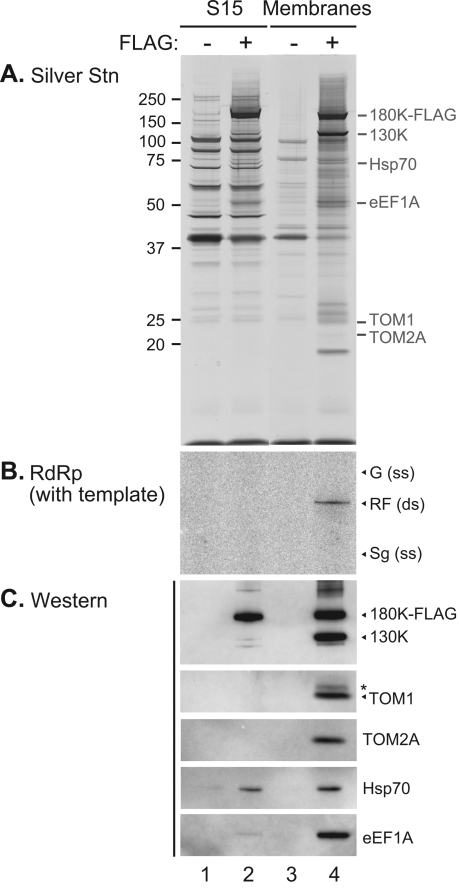
FIG. 8.
Affinity purification of FLAG-tagged 180K replication protein. The iBYL S15 fraction (lanes 1, 2) or iBYL membranes (lanes 3, 4) from BY-2 cells were treated with LPC and subjected to affinity purification with anti-FLAG antibody-conjugated agarose beads, as described in Materials and Methods. In the BY-2 cells, replication of a ToMV derivative encoding either 180K-FLAG (lanes 2, 4), or nontagged 180K proteins (lanes 1, 3) was occurring. (A) Silver staining of affinity-purified proteins separated by SDS-12% PAGE. Equal volumes of purified samples were loaded in each lane. The positions and masses (in kDa) of protein markers are shown on the left. The expected locations of 180K-FLAG, 130 K, TOM1, TOM2A, eEF1A, and Hsp70 are shown on the right. (B) RdRp activity in the presence of exogenous ToMV RNA template. For RdRp product abbreviations, see the legend to Fig. 2A (the single-stranded genomic and subgenomic RNA bands were not detected here). (C) Detection of ToMV replication proteins, the TOM1 and TOM2A proteins, Hsp70, and eEF1A in the purified fractions. In panels B and C, the samples in lanes 1, 2, 3, and 4 were applied in a volume ratio of 1:1:4.3:4.3, respectively. This ratio yielded similar intensities of the 180K-FLAG bands on Western blots in lanes 2 and 4. The abbreviations are described in the legends to Fig. 2A and D for RdRp and Western analyses, respectively.