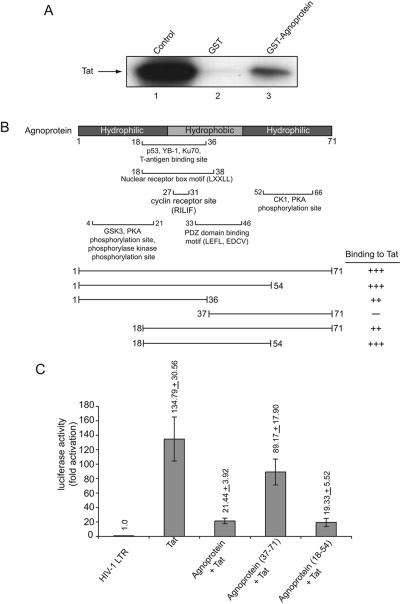
FIG. 5.
Physical and functional interaction of agnoprotein and Tat. (A) In vitro-synthesized 35S-labeled full-length Tat was incubated with either GST or GST-agnoprotein (full-length; amino acids 1 to 71) (55) immobilized on glutathione-Sepharose beads. Beads were washed extensively, and the protein complexes associated with GST or GST-agnoprotein were resolved by SDS-PAGE and analyzed by autoradiography. (B) For mapping, in vitro-synthesized 35S-labeled full-length Tat was incubated with either GST, GST-agnoprotein (1-71), or the deletion mutants of agnoprotein (as indicated) fused to GST and immobilized on glutathione-Sepharose beads. The bound proteins were analyzed by SDS-PAGE followed by autoradiography. (B) Structural organization of agnoprotein illustrating the various domains of agnoprotein. The ability of agnoprotein and its deletion mutants to interact with Tat is depicted on the right as follows: +++, strong interaction; ++, reduced interaction; +, weak interaction; and −, no interaction. (C) Effect of agnoprotein mutants on transcriptional activity of Tat. Primary human fetal astrocytes (2 × 105 in 60-mm plate) were transfected using FuGENE 6 transfection reagent with 1 μg of full-length (−450 to +80) HIV-LTR fused to the luciferase gene in the absence or presence of plasmids encoding Tat (1 μg) and full-length agnoprotein (1-71) (1 μg) and deletion mutants which demonstrated strong binding activity (18-54) or no binding ability (37-71) to Tat. The concentration of DNA in each transfection mixture remained constant by adding pCDNA3. Forty hours after transfection, cells were harvested and luciferase enzymatic activity was measured. Average values of multiple experiments are presented as n-fold effects.