Abstract
Previous studies established that vaccinia virus could enter cells by fusion with the plasma membrane at neutral pH. However, low pH triggers fusion of vaccinia virus-infected cells, a hallmark of viruses that enter by the endosomal route. Here, we demonstrate that entry of mature vaccinia virions is accelerated by brief low-pH treatment and severely reduced by inhibitors of endosomal acidification, providing evidence for a predominant low-pH-dependent endosomal pathway. Entry of vaccinia virus cores into the cytoplasm, measured by expression of firefly luciferase, was increased more than 10-fold by exposure to a pH of 4.0 to 5.5. Furthermore, the inhibitors of endosomal acidification bafilomycin A1, concanamycin A, and monensin each lowered virus entry by more than 70%. This reduction was largely overcome by low-pH-induced entry through the plasma membrane, confirming the specificities of the drugs. Entry of vaccinia virus cores with or without brief low-pH treatment was visualized by electron microscopy of thin sections of immunogold-stained cells. Although some virus particles fused with the plasma membrane at neutral pH, 30 times more fusions and a greater number of cytoplasmic cores were seen within minutes after low-pH treatment. Without low-pH exposure, the number of released cores lagged behind the number of virions in vesicles until 30 min posttreatment, when they became approximately equal, perhaps reflecting the time of endosome acidification and virus fusion. The choice of two distinct pathways may contribute to the ability of vaccinia virus to enter a wide range of cells.
There are two major infectious forms of vaccinia virus (VACV) with different outer membranes (24). The most abundant form, known as the intracellular mature virion (MV), consists of a nucleoprotein core surrounded by a lipid membrane containing more than 20 viral proteins. Following virus assembly, progeny MVs accumulate within the cytoplasm and may be released by cell lysis to infect other cells. Some MVs, however, are wrapped by modified trans-Golgi or endosomal cisternae and transported through the cytoplasm via microtubules to the plasma membrane, where exocytosis occurs (25, 33). The extracellular virion (EV), which is essentially an MV with an extra membrane containing at least six unique viral proteins, may remain on the parental cell surface or dissociate to mediate spread to adjacent or more-distal cells, respectively (5, 27). Because of its abundance, ease of purification, and stability, the MV is the form of VACV that is commonly used in laboratory and animal studies.
Entry of enveloped viruses usually involves three steps: cell attachment, activation of a fusion protein, and merging of viral and cellular membranes. The VACV MV membrane proteins A27, H3, and D8 were proposed to mediate initial attachment of MV to host cell glycosaminoglycans (8, 17, 21), though the importance of this may depend on the cell type (6). EVs bind to cells differently from MVs (36), but no EV binding protein has yet been demonstrated. Six putative entry/fusion protein components of the MV membrane were identified based on the phenotypes of conditional lethal null mutants (26, 30, 32, 34, 35; S. Ojeda et al., submitted for publication). In each case, normal-appearing virus particles that could bind to cells but not enter them or mediate cell-cell fusion were assembled under nonpermissive conditions. The associated finding that efficient cell-to-cell spread of the mutant viruses is inhibited under nonpermissive conditions provides genetic and biochemical evidence that fusion of the MV membrane is also necessary for EV entry. These six MV membrane proteins, as well as two more that have not yet been characterized with regard to function, assemble to form a putative fusion complex (31). The roles of the individual proteins and the mechanism of fusion remain to be elucidated. Another protein, A27, was long thought to have a role in membrane fusion mainly because the latter was blocked by a specific monoclonal antibody (MAb) (16). Subsequent analysis of inducible and null mutants, however, demonstrated that A27 is dispensable for virus entry and membrane fusion (29, 30, 38).
Enveloped viruses generally enter cells by fusion with the plasma membrane or by a low-pH endosomal route (15). Several studies indicated that MVs could enter cells by direct fusion with the plasma membrane at neutral pH (1, 6, 7, 10, 19, 36), although other entry mechanisms, including internalization (9, 28) and nonfusion mechanisms (22), were proposed. A recent study showed that EVs also could enter through the plasma membrane at neutral pH following disruption of the outer membrane and fusion of the released MV (20). Earlier, however, a role for endocytosis and low-pH-induced EV membrane disruption followed by MV membrane fusion was reported (18, 36). Thus, nearly all published data are consistent with the idea that only the MV membrane is fusogenic, but there is uncertainty regarding the entry pathways of the MV and EV.
Virus-induced cell-cell fusion is a process that is usually related to virus entry. VACV is capable of inducing syncytium formation when cells with progeny EVs or adsorbed MVs bound to the cell surface are briefly acidified and then returned to neutral pH (5, 10, 16). Although neither the significance nor the mechanism of low-pH-triggered syncytium formation induced by VACV has been investigated, for other viruses, this behavior is thought to reproduce the fusion reaction that occurs during entry through acidified endosomes. If VACV enters cells exclusively by direct fusion with the plasma membrane at neutral pH, the possession of a mechanism for inducing syncytia after acidic-pH treatment would be inexplicable. It seems likely, therefore, that the low-pH fusion mechanism is used by poxviruses for cell entry, an idea that was reinforced by the determination that low-pH-triggered syncytium formation is dependent on each of six proteins essential for MV entry (26, 30, 32, 34, 35; Ojeda et al., submitted). Accordingly, we decided to examine the effects of low-pH treatment and of preventing the acidification of endosomes on VACV entry. Our studies indicate that a large fraction of MVs enter through a low-pH-dependent endosomal route. The ability of VACV to enter cells by both neutral-pH plasma membrane and low-pH endosomal pathways may contribute to its wide host range in cell culture.
MATERIALS AND METHODS
VACV gene and protein nomenclature.
All experiments were performed with the Western Reserve (WR) strain of VACV (ATCC VR-1354) or with mutant viruses derived from this strain. VACV WR genes (GenBank accession number AY243312) are numbered consecutively from the left to the right end of the genome. A common nomenclature for VACV genes used for the Copenhagen strain of VACV, consisting of the letter designation for the HindIII fragment, the number of the open reading frame within that fragment, and L or R to denote direction of transcription, was also used for WR in the present report. For the corresponding gene products, the L and R designations were omitted.
Purification of VACV and infection of cells.
VACV MVs were isolated by mechanical disruption of HeLa S3 cells, purified by sedimentation twice through a 36% sucrose cushion and banded once on a 25 to 40% sucrose gradient, and titrated by plaque assay on BS-C-1 cells as described previously (13, 14). Purified MV stocks were stored at −80°C and sonicated on ice three times for 1 minute each upon thawing. Virus was adsorbed to cells passively at 4°C for 1 h in Earle's minimal essential medium (EMEM; Quality Biologicals) containing 2.5% fetal bovine serum unless otherwise indicated. The cells were then washed with cold EMEM with 2.5% serum and overlaid with prewarmed 37°C phosphate-buffered saline (PBS), pH 7.4, or PBS that had been adjusted to lower pH with HCl and 1 mM 2-morpholinoethanesulfonic acid. After 3 to 5 min, the cells were washed and overlaid with EMEM containing 2.5% serum.
Recombinant VACV construction.
DNA encoding firefly luciferase (GenBank accession number AAL30790) was amplified by PCR (Accuprime Pfx; Invitrogen), purified, and cloned into pRB21 (4) to generate pRB21Fire. BS-C-1 cells were infected with 1 PFU per cell of vRB12 (4) in Optimem medium (Invitrogen) and 2 h later were transfected with 0.3 μg of pRB21Fire by using Lipofectamine 2000 (Invitrogen). Infected cells were harvested 24 h after infection and freeze- thawed three times, and dilutions were replated onto BS-C-1 monolayers. The desired recombinant virus WRvFire was distinguished from the parental vRB12 by the formation of large plaques and was clonally purified by five rounds of plaque isolation.
Western blot analysis.
VACV WR-infected BS-C-1 cells in EMEM were harvested at various times, washed in PBS, and disrupted in lithium dodecyl sulfate sample buffer (Invitrogen) containing NuPage reducing agent (Invitrogen). Lysates were sonicated and incubated at 100°C for 4 min and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a 4 to 12% NuPage Bis-Tris gel (Invitrogen). Proteins were transferred to a nitrocellulose membrane and blocked with Tris-buffered saline supplemented with 5% nonfat dried milk and 0.05% Tween 20 for 1 h at room temperature. Subsequently, the membrane was incubated with rabbit anti-VACV serum (12), washed, and incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Pierce). Western blots were analyzed using chemiluminescence reagents (Pierce).
Firefly luciferase assays.
Virus was adsorbed to cells in 12-well plates for 1 h at 4°C, unattached virus was removed by washing, and cells were subjected to a 3- to 5-min incubation at 37°C with buffer of the desired pH. After being washed, cells were incubated in 1 ml of EMEM with or without inhibitor at 37°C. Cells were harvested after being washed with PBS, pH 7.4, and incubated in 300 μl of cell culture lysis reagent (Promega) for 30 min at room temperature with gentle agitation. A 20-μl portion of the resulting lysate was mixed thoroughly with 100 μl of luciferase assay substrate (Promega), and chemiluminescence was quantified on a Berthold Sirius luminometer.
Inhibition of endosomal acidification.
Monkey BS-C-1, rabbit RK-13, or human HuTK− (thymidine kinase-deficient) cells seeded in 12-well plates were pretreated with bafilomycin A1, concanamycin A, or monensin from Sigma in EMEM or mock treated for 50 min at 37°C. Following this treatment, cells were rapidly chilled to 4°C before the addition of virus to the existing medium. Virus was adsorbed to the cells for 1 h at 4°C and then washed with ice-cold EMEM to remove unattached virus before incubation for 3 min at 37°C with either pH 5.0 or pH 7.4 buffer. Cells were then washed with EMEM and incubated at 37°C in the presence of inhibitor for the duration of the experiment.
Confocal microscopy.
Infected BS-C-1 cells were fixed with 3% paraformaldehyde in PBS for 20 min at room temperature, washed three times with PBS, and quenched with 2% glycine. Cells were incubated with or without Alexa Fluor 568-conjugated phalloidin (Invitrogen) diluted in 10% complement-inactivated fetal bovine serum in PBS for 1 h at 37°C. Cells were washed three times with PBS prior to incubation with diamidino-2-phenylindole dihydrochloride (DAPI; Invitrogen) to visualize DNA. Images were obtained using a Leica TCS-NT/SP2 inverted confocal microscope with an attached argon laser (Coherent Inc.).
Immunoelectron microscopy.
A suspension of purified MVs (300 PFU/cell) in 1 ml of EMEM was applied to a BS-C-1 monolayer in a six-well tissue culture plate that had been equilibrated for 30 min at 4°C. The plate was wrapped in flexible polyvinylidene chloride (Saran Wrap) and placed in a 75006449 C bucket in a Heraeus rotor in a Legend RT centrifuge from Sorvall. The virus was pelleted onto the cells at 650 × g for 1 h at 4°C. Subsequently, the samples were fixed on ice with 4% paraformaldehyde in 0.1 M phosphate buffer (Electron Microscopy Sciences, Ft. Washington, PA) for 10 min, followed by 8% paraformaldehyde for 50 min at room temperature, and quenched for 20 min with 20 mM glycine in 0.1 M phosphate buffer (6). The samples were then blocked in 0.1% fish skin gelatin in 0.1% phosphate buffer (Sigma, St. Louis, MO) for 5 min and incubated with a 1:100 dilution of mouse MAb AB1.1 (anti-D8, provided by G.L. Smith), washed in 0.1% fish skin gelatin followed by incubation with a 1:500 dilution of rabbit anti-mouse antibody (ICN Pharmaceuticals, Inc. Aurora, OH), and finally incubated with protein A conjugated to 5 nm colloidal gold (Dept. of Cell Biology, Utrecht University School of Medicine, Utrecht, Netherlands). The samples were fixed for 1 h with 2% glutaraldehyde in 0.1% phosphate buffer (Electron Microscopy Sciences) and washed once in phosphate buffer and then several more times in 0.1 M sodium cacodylate buffer. The samples were postfixed in reduced osmium tetroxide in 0.1 M sodium cacodylate buffer, washed in buffer, and then dehydrated in a series of ethyl alcohol and propylene oxide solutions. The samples were embedded in Embed 812 (Electron Microscopy Sciences) and sectioned with a Leica Ultracut S ultramicrotome. Thin sections were stained with 7% uranyl acetate in 50% ethanol followed by 0.01% lead citrate. Sections were reviewed and photographed under a Philips (FEI Company) CM100 transmission electron microscope fitted with a Gatan charge-coupled-device camera.
RESULTS
Brief low-pH exposure accelerates VACV entry.
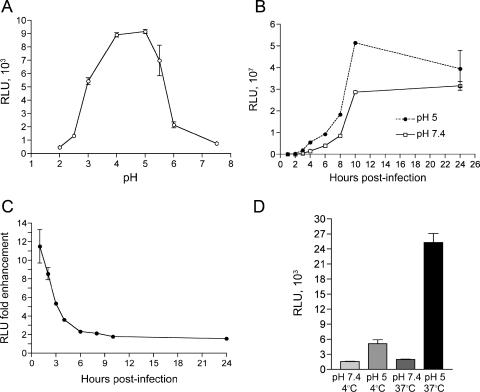
Viral gene expression occurs in the cytoplasm soon after entry of poxviruses because the entire early transcription system is incorporated within the core and poised to function after removal of the membrane. Therefore, early gene expression can serve as an indicator of an early postfusion event. For quantification purposes, the recombinant virus WRvFire, containing the firefly luciferase gene regulated by a strong VACV early-late promoter, was constructed. To determine the effect of pH on entry, purified MVs were adsorbed to cells at 4°C and the latter were then immersed in buffers of varied pH for 5 min. After an additional hour at neutral pH, the cells were assayed for luciferase. As shown in Fig. 1A, luciferase production was strongly enhanced after brief incubation at pH values below 6, with a >10-fold enhancement at pH 4.0 to 5.5.
FIG. 1.
Effect of brief low-pH treatment on MV entry as determined by a luciferase reporter assay. (A) pH optimum. BS-C-1 cell monolayers were inoculated with WRvFire MVs at a multiplicity of 5 PFU per cell at 4°C for 1 h. After being washed, cells were treated with buffer at the indicated pH values for 3 min at 37°C, washed again with EMEM, incubated for 1 h at 37°C in EMEM, and then lysed. Firefly luciferase activity was determined by chemiluminescence. Experiments were performed in duplicate, and data points represent the means ± standard errors. (B) Time course of luciferase accumulation. BS-C-1 cell monolayers were treated with AraC for 1 h at 37°C prior to passive inoculation with WRvFire MVs at a multiplicity of 0.2 PFU per cell at 4°C for 1 h. After being washed, cells were treated with pH 5.0 or pH 7.4 buffer for 3 min at 37°C. Cells were washed with EMEM and incubated at 37°C. At the indicated times, cells were lysed and synthesis of firefly luciferase was determined. Experiments were performed in duplicate, and data points represent the means ± standard errors. Note the large difference in scale between panels A and B. (C) Data from panel B were plotted as the ratios of activities after low- and neutral-pH treatments. (D) Temperature requirement. Cells were inoculated with WRvFire MVs as in panel A and treated with pH 5.0 or pH 7.4 buffer for 3 min at either 4°C or 37°C. Cells were washed and incubated with EMEM for 1 h at 37°C and assayed for luciferase activity as in panel A. RLU, relative light units.
Further experiments indicated that luciferase activity increased greatly with time and that the effect of low pH was mainly to accelerate entry. In the experiment depicted in Fig. 1B, the DNA synthesis inhibitor AraC was present so that viral mRNA would be made only from the input cores and not from replicated genomes. Because the scale of luciferase activity is 10,000-fold greater than that in Fig. 1A, the values at very early times cannot be discerned. To clearly assess the effect of low pH, the ratios of luciferase activities from cells treated with pH 5.0 and 7.4 buffers were plotted versus time (Fig. 1C). At 1 h, luciferase activity was elevated 11.5-fold in cells treated with pH 5.0 buffer with respect to that in cells treated with pH 7.4 buffer. The enhancing effect of pH diminished over time, so that by 24 h postinfection, the luciferase activity in pH 5.0-treated cells was only 1.4 times greater than that in the pH 7.4-treated control.
Our standard procedure was to incubate virions with cells at 4°C, a temperature that permits attachment but not entry, and then perform the low-pH treatment at 37°C. We found that if the low-pH treatment was carried out at 4°C rather than 37°C, the enhancing effect was minimal (Fig. 1D).
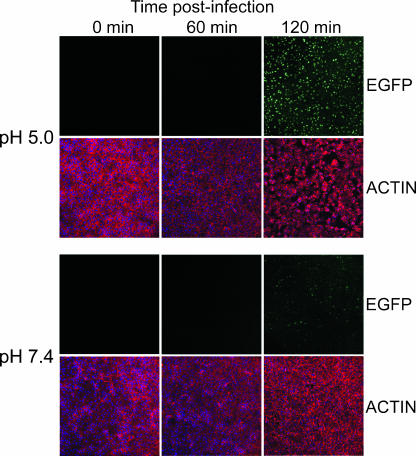
In the above-described experiments, it was possible that low pH greatly enhanced expression in only a minor fraction of the infected cells. To evaluate this, MVs from a recombinant VACV that expresses enhanced green fluorescent protein (EGFP) under the control of an early-late promoter were adsorbed to BS-C-1 cells at 4°C; the cells were then exposed briefly to either pH 7.4 or pH 5.0 buffer and incubated further with regular medium at neutral pH. Bound MVs exposed to acidic pH initiated a rapid and synchronous infection, as evidenced by uniform fluorescence in all cells by 2 h postinfection (Fig. 2). In contrast, the fluorescences of infected cells that were exposed to pH 7.4 buffer were variable and considerably less intense at 2 h (Fig. 2). Rounding and retraction of cells, indicators of a cytopathic effect mediated by VACV early gene products, were evident by actin staining at 2 h only after exposure to low pH (Fig. 2). The cytopathic effect did not occur in mock-infected cells that were exposed to low pH for the same time (data not shown), confirming that altered cell morphology required VACV infection.
FIG. 2.
Synchronization of infection by low-pH treatment. BS-C-1 cell monolayers were passively inoculated with EGFP-expressing MVs at a multiplicity of 5 PFU per cell at 4°C for 1 h. After being washed, cells were treated with pH 5.0 or 7.4 buffer for 3 min at 37°C, washed and incubated in EMEM for 0, 60, or 120 min at 37°C, fixed with 3% paraformaldehyde, and quenched with 2% glycine. Cells were stained with Alexa Fluor 568-conjugated phalloidin to visualize filamentous actin and examined by confocal microscopy. Actin, red; EGFP fluorescence, green.
Low-pH exposure hastens the progression of a productive VACV infection.
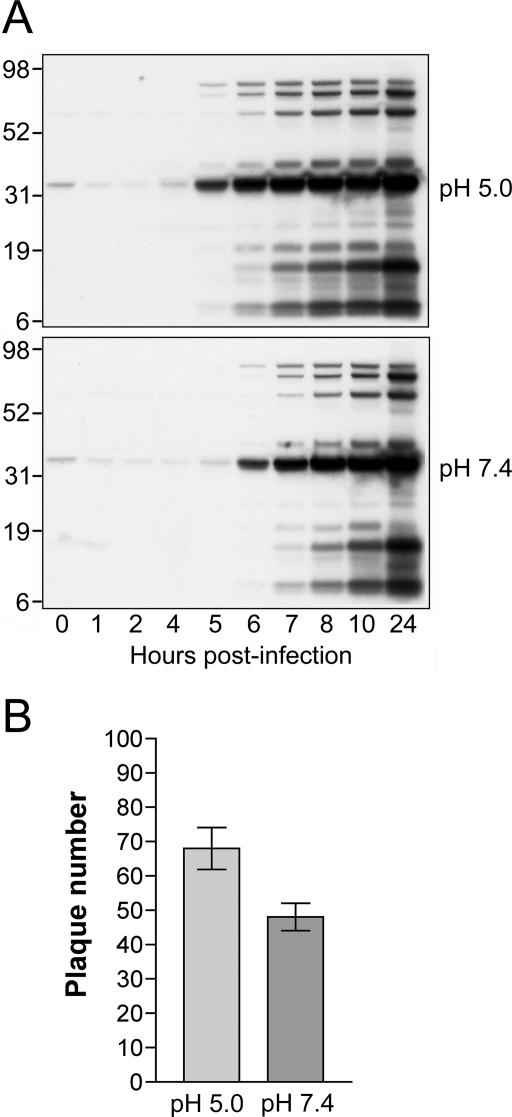
Further experiments were carried out to determine whether accelerated entry of MVs at low pH leads to a productive or unproductive infection. After low-pH treatment, synthesis of viral proteins was determined by Western blotting with polyclonal antibodies recognizing abundant VACV late proteins. As shown in Fig. 3A, the same viral late protein pattern was detected whether low- or neutral-pH buffer was used. However, viral proteins were detected at least 1 h earlier, when low-pH treatment was employed, consistent with accelerated entry. We also compared the efficiency of plaque formation when virus adsorbed to a cell monolayer was exposed to pH 5.0 or 7.4. Low-pH treatment increased the number of plaques 1.4-fold (Fig. 3B). Thus, brief low-pH treatment of cells following virus adsorption leads to accelerated entry and a productive VACV infection.
FIG. 3.
Low-pH treatment enhances a productive virus infection. (A) Western blots comparing VACV late gene expression following low- and neutral-pH treatments. BS-C-1 cell monolayers were incubated with purified MVs at 5 PFU per cell at 4°C for 1 h. After being washed, cells were treated with pH 5.0 or pH 7.4 buffer for 3 min at 37°C, washed with EMEM, and incubated at 37°C. At the indicated times, cell lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and analyzed by Western blotting with an anti-VACV antiserum. The positions and molecular masses in kDa of marker proteins are shown on the left. (B) Effect of pH treatment on plaque formation. BS-C-1 cell monolayers in six-well plates were inoculated with approximately 50 PFU per well of purified MVs at 4°C for 1 h. After removal of unbound virus by washing, cells were treated with pH 5.0 or pH 7.4 buffer for 3 min, washed with EMEM, overlaid with 2% methylcellulose in EMEM, and incubated at 37°C. At 48 h postinfection, monolayers were fixed and stained with crystal violet and plaques counted. Experiments were performed in triplicate, and data points represent the means ± standard errors.
Low pH triggers fusion of MV and plasma membranes.
The reporter gene expression experiments measured an early postentry event. Electron microscopy was employed to directly visualize virus fusion with the plasma membrane. Because the passive binding of MVs to cells is relatively slow and asynchronous, a low-speed centrifugation procedure referred to as spinoculation has been used to deposit large numbers of VACV particles on the cell surface for electron microscopy (6). Before adapting spinoculation for our studies, we established that the procedure increased entry and early gene expression as measured by a 30- to 50-fold increase in luciferase activity at 1 h after virus infection at neutral pH.
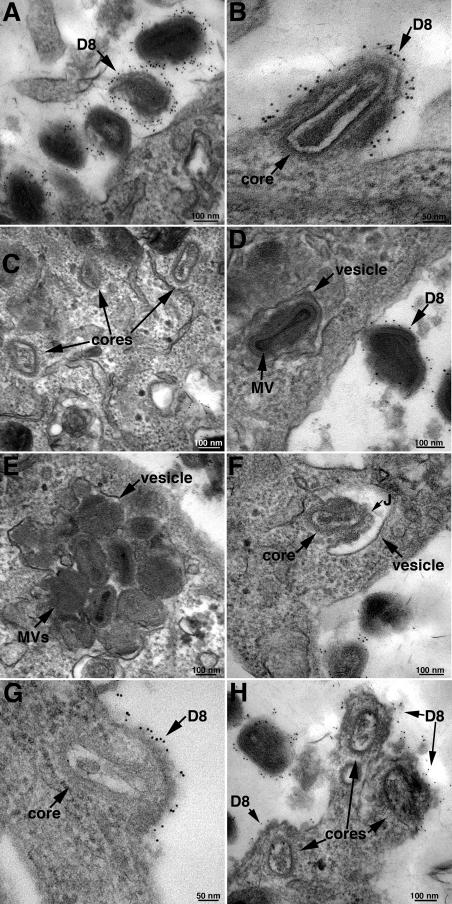
For electron microscopy, spinoculated BS-C-1 cells were exposed to pH 7.4 or 5.0 buffer at 37°C for 5 min, washed, and incubated in regular neutral-pH medium. Zero, 10, 20, or 30 min later, the cells were fixed without permeabilization and incubated with a MAb to the viral D8 membrane protein followed by a secondary antibody and protein A conjugated to gold grains essentially as previously described (6). This procedure allowed us to distinguish antibody-labeled virions outside the cell from unlabeled virions in vesicles inside the cell and to detect fusion of viral and plasma membranes by D8 labeling. Results obtained with neutral-pH treatment are discussed in the remainder of this paragraph. Numerous extracellular MVs labeled with gold grains were present at each of the times examined as shown for the 10-min time point in Fig. 4A. Examples of an individual core beneath a segment of plasma membrane that was labeled by D8 MAb were found at each of the times, including the first 10 min (Fig. 4B). However, 90% of the cores were not in proximity to the D8-staining plasma membrane (Fig. 4C), suggesting that fusion with the plasma membrane was not the main route of entry or that the D8 protein diffused away very quickly. The number of cytoplasmic cores increased greatly between 20 and 30 min but without a concomitant increase in observable plasma membrane fusions as quantified below. Unlabeled virions were present in cytoplasmic vesicles at 10 min and increased in number over the next 20 min. Vesicles containing single (Fig. 4D) and multiple (Fig. 4E) virions were observed at each time point. Rarely, a virion was seen that appeared to be fusing with the vesicle membrane (Fig. 4F).
FIG. 4.
Immunogold electron microscopy. BS-C-1 cells were spinoculated with 300 PFU/cell of purified MVs and exposed to pH 5.0 or 7.4 buffer for 5 min at 37°C. The cells were further incubated in EMEM for 0, 10, 20, or 30 min. At each time point, the unpermeabilized cells were stained with a MAb to the VACV D8 membrane protein followed by anti-mouse MAb and protein A conjugated to 5-nm gold spheres. The cells were then cryosectioned and examined by electron microscopy. (A) Neutral pH, 10-min time point; immunogold-labeled MVs at the cell surface. (B) Neutral pH, 10-min time point; MV fusing with the plasma membrane. (C) Neutral pH, 10-min time point; intracellular cores not associated with immunogold-labeled plasma membrane. (D) Neutral pH, 20-min time point; MV in cytoplasmic vesicle (note absence of gold grains in particle within vesicle and numerous grains in a nearby particle at the cell surface). (E) Neutral pH, 20-min time point; multiple MVs in cytoplasmic vesicle. (F) MV apparently fusing with the vesicle membrane within the cytoplasm. The putative junction between the vesicle and viral membrane is indicated by the letter J. (G) Low pH, 10-min time point; MV fusing with the plasma membrane. (H) Low pH, 10-min time point; three cores are seen with diffuse gold staining of nearby plasma membranes, suggesting that some time had elapsed after the fusion events. Bars indicate magnification.
At 10 min after low-pH treatment, there were many cores in the cytoplasm and patches of D8-labeled plasma membrane were in the proximity of more than 60% of them, indicating a recent fusion event (Fig. 4G and H). The number of cytoplasmic cores did not increase at 20 or 30 min, as quantified below, but at these times, most were no longer near the D8-labeled plasma membrane. Assuming that the cores are stable during this period, this result suggested that the majority had entered during the first 10 min and that the D8 patches in the plasma membrane had diffused by 20 min. There were fewer virus particles in cytoplasmic vesicles at either 10 or 20 min after low-pH treatment than after neutral-pH treatment, but the number increased at 30 min. The delay could be due to the low-pH-triggered fusion of those virions poised to enter by endocytosis and the time needed for additional virions to more closely attach to the plasma membrane. Alternatively, the cells might have had to recover from the low-pH shock before virus entry by endocytosis could occur.
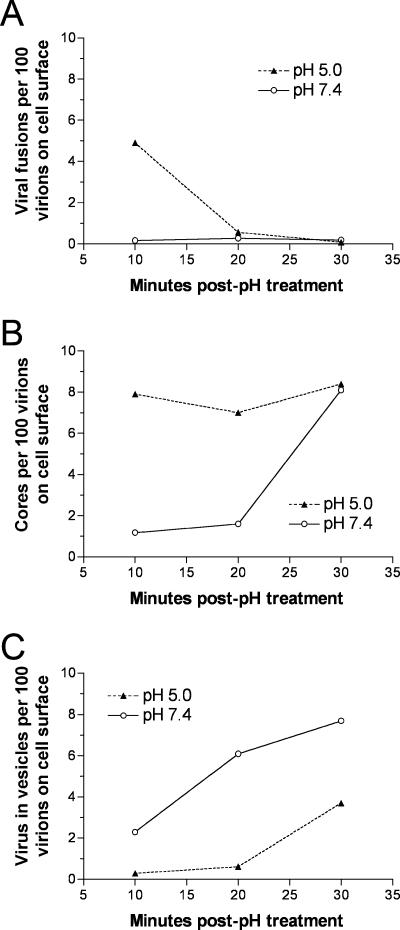
To quantify the data, in each cell section, we counted (i) the total number of intracellular cores, (ii) the subset of cores in proximity with a D8-staining plasma membrane indicative of a recent fusion event, and (iii) the total number of nonstaining virus particles in cytoplasmic vesicles. We also counted the number of extracellular virions in the same fields in order to normalize the data. As large numbers of extracellular virions were present throughout the 30-min period, no correction for their depletion by entry was made. By comparing the data obtained with neutral- and low-pH treatments, we verified that the latter enhanced fusion events at the plasma membrane 30-fold during the first 10 min (Fig. 5A), accelerated entry of cores into the cytoplasm during the same time (Fig. 5B), and delayed endocytosis of virus particles (Fig. 5C). Moreover, after low-pH treatment, there was a strict correlation between time of core entry and plasma membrane fusions. In the absence of low-pH exposure, the numbers of cytoplasmic cores increased significantly at 30 min, perhaps reflecting the time of endosome acidification and virus fusion. Thus, the microscopy data provided an explanation for the accelerated expression of luciferase following low-pH treatment and suggested that neutral-pH entry was largely endosomal.
FIG. 5.
Quantification of images visualized by immunogold electron microscopy. Data are from the same experiment used to obtain images in Fig. 4. In each cell section, the number of intracellular cores, the subset of cores in proximity with a D8-staining plasma membrane indicative of a recent fusion event, and the number of nonstaining virus particles in cytoplasmic vesicles were counted. To normalize the data, each of the above-described numbers was divided by the number of extracellular virions in the same fields and multiplied by 100. (A) Plasma membrane fusion events. At 10, 20, and 30 min, there were 0.16, 0.27, and 0.19 fusions per 100 extracellular virions in the absence of low-pH treatment and 4.9, 0.56, and 0.05 after low-pH treatment, respectively. (B) Cytoplasmic cores. At 10, 20, and 30 min, there were 1.2, 1.6, and 8.1 cores per 100 extracellular virions without low-pH treatment and 7.9, 7.0, and 8.4 after low-pH treatment, respectively. (C) MVs in vesicles. At 10, 20, and 30 min, there were 2.3, 6.1, and 7.7 virions in vesicles per 100 extracellular virions without low-pH treatment and 0.29, 0.61, and 3.7 with low-pH treatment, respectively.
An experiment was carried out to differentiate between the steps of endocytosis and liberation of cores into the cytoplasm. Purified MVs lacking the A21 protein component of the entry-fusion complex (35) were processed for transmission electron microscopy at 30 min after adsorption, with omission of low-pH treatment. No free cores were detected, even though many virions were present in cytoplasmic vesicles. Therefore, the fusion complex is required for exit of cores from endosomal vesicles.
Inhibitors of endosomal acidification decrease MV entry.
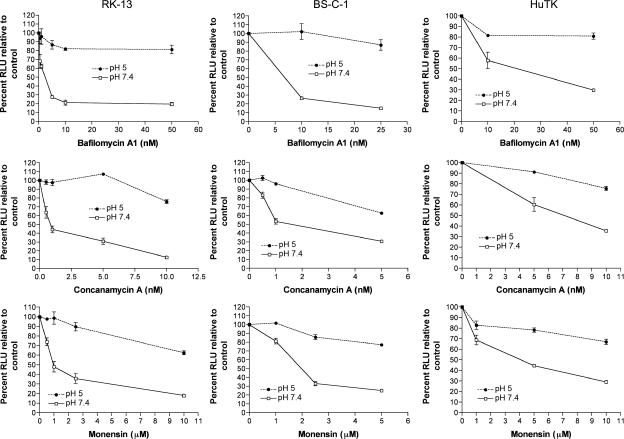
The increased fusion at the plasma membrane triggered by low pH and the presence of virus particles in cytoplasmic vesicles at early times after neutral pH treatment strongly suggested that VACV normally uses a low-pH endosomal pathway. If this interpretation were correct, we would expect inhibitors of endosomal acidification to reduce entry. For this study, we used bafilomycin A1 and concanamycin A, specific inhibitors of the vacuolar H+-ATPase (11), and monensin, a carboxylic ionophore that equilibrates protons between the cytosol and intracellular compartments to prevent endosomal acidification (23). To control for drug inhibition of postfusion events possibly affecting luciferase expression, MVs were adsorbed to drug-treated cells and incubated at low pH to induce MV fusion at the plasma membrane. Without low-pH treatment, we found that bafilomycin A1 and concanamycin A inhibited luciferase expression in a dose-dependent manner by more than 70% in a rabbit and a monkey cell line and to a slightly lesser degree in one human cell line (Fig. 6). Comparable inhibition also occurred when spinoculation was used for infection (data not shown). As predicted, inhibition of virus entry resulting from bafilomycin A1 and concanamycin A treatments could be largely prevented by inducing entry at the plasma membrane with low pH, thereby obviating the need for low-pH endosomes (Fig. 6). The small reductions after low-pH treatment could result from some entry by endocytosis, as suggested by electron microscopy of cells at 30 min after adsorption (Fig. 4C), or represent a nonspecific effect. Results similar to those obtained with bafilomycin A1 and concanamycin were obtained following treatment with monensin (Fig. 6). Therefore, inhibition of endosomal acidification by two distinct mechanisms resulted in comparable reductions in virus entry. These data indicate that a large fraction of VACV WR MVs penetrate cells through a low-pH-dependent endosomal pathway.
FIG. 6.
Inhibitors of endosomal acidification prevent VACV entry. BS-C-1, RK-13, and HuTK− cell monolayers were pretreated with the indicated inhibitors for 50 min at 37°C. Following this incubation, the cells were chilled to 4°C and then inoculated with WRvFire MVs at a multiplicity of 5 PFU per cell at 4°C for 1 h. After removal of unadsorbed virus, cells were treated with either pH 5.0 or pH 7.4 buffer for 3 min at 37°C. Cells were washed with EMEM and incubated for 1 h at 37°C in the presence of inhibitor. Cells were lysed, and synthesis of firefly luciferase was determined by chemiluminescence. Experiments were performed in duplicate, and data points represent the means ± standard errors. RLU, relative light units.
DISCUSSION
The majority of enveloped viruses enter cells either by neutral-pH fusion with the plasma membrane or by endocytosis followed by low-pH-dependent fusion with the endosomal membrane. Here, we show that VACV uses a low-pH endosomal mechanism in addition to the previously described neutral-pH entry, a versatility that may contribute to its wide host range. Our conclusion that MVs use a low-pH endosomal pathway was based on findings that entry was accelerated and inhibited by brief acid treatment and prevention of endosomal acidification, respectively. The presence of the early transcription system within poxvirus cores allowed us to analyze events closely following the entry step by using recombinant VACVs that express EGFP or firefly luciferase from early promoters. An enhancing effect on luciferase synthesis occurred between pH 5.5 and 4.0 and was greatest at early times after infection; at 1 h, the stimulation by pH 5 treatment was >11-fold, whereas it was <1.5-fold at 24 h. In accord with this, we found that low-pH treatment only modestly hastened late gene expression and plaque formation. The pH 5.5 threshold for activation was similar to that found for plasma membrane fusion of other viruses that use the endosomal route of entry, including Semliki Forest virus, vesicular stomatitis virus, and influenza virus (39). The low-pH enhancement of VACV entry was temperature dependent, as it was slight when this step was carried out at 4°C. Further studies are needed to determine the molecular mechanism of low-pH enhancement of VACV entry.
Events associated with MV entry were visualized by electron microscopy in conjunction with immunogold labeling. Antibody to the VACV D8 membrane protein served as a marker for fusion with the plasmalemma and also allowed us to differentiate labeled extracellular virions from unlabeled virions that were endocytosed prior to labeling. Low pH triggered the rapid (<10 min) and synchronous fusion of MVs with the plasma membrane and the deposition of free cores in the underlying cytoplasm. There was a strict correlation between time of core entry and plasma membrane fusion. In the absence of low-pH treatment, 30-fold fewer plasma membrane fusion events were detected and free cores accumulated more slowly. Under the latter conditions, the numbers of free cores lagged behind the numbers of virions in vesicles until 30 min posttreatment, when they became approximately equal, perhaps reflecting the time of endosome acidification and virus fusion. MVs in the act of fusing with the vesicle membrane were seen only rarely, however, probably due to the asynchrony of entry by this means and the rapidity of the process once the endosome is acidified. The microscopic observations explained the biochemical data, indicating that low pH enhanced fusion with the plasma membrane and that otherwise, entry occurs via a predominant endocytic route in addition to fusion with the plasma membrane. We also determined that components of the entry-fusion complex are required for core entry but not for endocytosis of MVs.
The effect of pHs of <6 on VACV entry was not previously investigated by confocal or electron microscopy. The dramatic effect of low pH on entry of cores shown here was not revealed in the confocal microscopy study of Vanderplasschen et al. (36) because pH values below 6 were not tested. Several investigators used electron microscopy to study entry of VACV at neutral pH but interpreted their results exclusively in favor of one mechanism or another. Dales (9) demonstrated that MVs were taken up by phagocytic vacuoles within an hour after adsorption and considered this the normal entry route. Subsequently, others (1, 7) found that fusion of MVs with the plasma membrane occurred earlier and concluded that this was the biologically relevant mechanism. Considering similar data, Payne and Norrby (28) argued oppositely that because plasma membrane fusion could be detected only during the first 15 min after adsorption, a time at which a minority (∼35%) of virions had acquired resistance to neutralizing antibody, the slower process of endocytosis was more important. Carter et al. (6) confirmed the fusion of MVs with the plasma membrane but did not consider other modes of entry. By showing that entry occurs both by endocytosis and by fusion with the plasma membrane, our data bring together previous seemingly disparate observations.
Brief low-pH treatment can trigger the fusion of cells with adsorbed virus inoculum or progeny virus particles on their cell surface (2, 16). The role of low pH in triggering cell-cell fusion, however, was unexplained. We found that the pH optimum for fusion of MVs with the plasma membrane was similar to that previously found for cell-cell fusion (16), suggesting that the two processes are related. Syncytia could form either by simultaneous fusion of virions that are bridging two cells or by a stepwise deposition of viral proteins into the plasma membrane of one cell followed by its fusion with a neighboring cell. In this context, we found that low-pH treatment of free virions does not inactivate them (our unpublished data).
In addition to showing that low pH enhances plasma membrane fusion, we demonstrated that inhibitors of endosomal acidification reduced MV entry in a dose-dependent manner by more than 70%. Similar results were obtained with bafilomycin A1 and concanamycin A, two different inhibitors of the vacuolar H+-ATPase (11), and monensin, a carboxylic ionophore that equilibrates protons between the cytosol and intracellular compartments to prevent endosomal acidification (23). Because we used an indirect luciferase assay, it was important to show that these drugs were specific for endosomal entry and not for downstream steps related to luciferase expression. Specificity was demonstrated by showing that these drugs had a minimal effect on luciferase activity when entry through the plasma membrane was triggered by low pH, thereby bypassing the endosomal pathway. In contrast, we found that the lysosomotropic weak bases NH4Cl and chloroquine reduced luciferase expression after both neutral- and low-pH treatments (A. C. Townsley, unpublished), suggesting that they have postentry effects as has been found in another virus infection system (37). Using an assay that depends on the inability of intact MV particles to be stained with a core antibody, Vanderplasschen et al. (36) found only a small effect of NH4Cl or chloroquine on formation of cores. This result may be due to their use of the IHD-J strain of VACV, since we found that this strain is also resistant to bafilomycin and monensin (A. C. Townsley, unpublished). We are currently carrying out experiments to determine the genetic and biochemical basis for the apparent difference between WR and IHD-J entry. Although the WR genome has been sequenced, the IHD-J genome has not, so the extent of divergence is unknown. One presumably unrelated difference is that EVs have a high rate of dissociation from the cell surface due to a mutation in the A34R gene of the IHD-J strain (3).
The greater-than-70% inhibition of MV entry by bafilomycin A1 suggests that a low-pH endosomal pathway is the major route of entry at least for the virus strain and cell lines used in this study. Whether the residual 30% signifies neutral-pH entry or incomplete drug inhibition was not ascertained. The electron microscopy results also suggested a predominant endosomal entry route, but the extent of plasma membrane fusion at neutral pH could not be accurately quantified because of asynchrony of entry under these conditions and the rapidity of the fusion event. Additional studies are also needed to evaluate the mode(s) of entry of the EV, as here too there are some reports that suggest a low-pH mechanism and others that favor neutral pH.
Acknowledgments
We thank Susan Parrish, Wolfgang Resch, and Erica Brown for helpful discussions, Norman Cooper for providing cells, Owen Schwartz for help with confocal microscopy, and John Heuser for discussions and comments on the manuscript.
This research was supported by the NIAID, NIH intramural program.
REFERENCES
- 1.Armstrong, J. A., D. H. Metz, and M. R. Young. 1973. The mode of entry of vaccinia virus into L cells. J. Gen. Virol. 21:533-537. [DOI] [PubMed] [Google Scholar]
- 2.Blasco, R., and B. Moss. 1991. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-dalton outer envelope protein. J. Virol. 65:5910-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blasco, R., and B. Moss. 1992. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J. Virol. 66:4170-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasco, R., and B. Moss. 1995. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene 158:157-162. [DOI] [PubMed] [Google Scholar]
- 5.Blasco, R., J. R. Sisler, and B. Moss. 1993. Dissociation of progeny vaccinia virus from the cell membrane is regulated by a viral envelope glycoprotein: effect of a point mutation in the lectin homology domain of the A34R gene. J. Virol. 67:3319-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter, G. C., M. Law, M. Hollinshead, and G. L. Smith. 2005. Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J. Gen. Virol. 86:1279-1290. [DOI] [PubMed] [Google Scholar]
- 7.Chang, A., and D. H. Metz. 1976. Further investigations on the mode of entry of vaccinia virus into cells. J. Gen. Virol. 32:275-282. [DOI] [PubMed] [Google Scholar]
- 8.Chung, C. S., J. C. Hsiao, Y. S. Chang, and W. Chang. 1998. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J. Virol. 72:1577-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dales, S. 1963. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J. Cell Biol. 18:51-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doms, R. W., R. Blumenthal, and B. Moss. 1990. Fusion of intra- and extracellular forms of vaccinia virus with the cell membrane. J. Virol. 64:4884-4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drose, S., and K. Altendorf. 1997. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200:1-8. [DOI] [PubMed] [Google Scholar]
- 12.Earl, P. L., J. L. Americo, and B. Moss. 2003. Development and use of a vaccinia virus neutralization assay based on flow cytometric detection of green fluorescent protein. J. Virol. 77:10684-10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earl, P. L., N. Cooper, and B. Moss. 1991. Preparation of cell cultures and vaccinia virus stocks, p. 16.16.1-16.16. 7. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Green Publishing, New York, N.Y. [Google Scholar]
- 14.Earl, P. L., and B. Moss. 1991. Generation of recombinant vaccinia viruses, p. 16.17.1-16.17.16. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Green Publishing, New York, N.Y. [Google Scholar]
- 15.Earp, L. J., S. E. Delos, H. E. Park, and J. M. White. 2005. The many mechanisms of viral membrane fusion proteins. Curr. Top. Microbiol. Immunol. 285:25-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong, S. C., C. F. Lai, and M. Esteban. 1990. Vaccinia virus induces cell fusion at acid pH and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology 178:81-91. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao, J. C., C. S. Chung, and W. Chang. 1999. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol. 73:8750-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichihashi, Y. 1996. Extracellular enveloped vaccinia virus escapes neutralization. Virology 217:478-485. [DOI] [PubMed] [Google Scholar]
- 19.Janeczko, R. A., J. F. Rodriguez, and M. Esteban. 1987. Studies on the mechanism of entry of vaccinia virus in animal cells. Arch. Virol. 92:135-150. [DOI] [PubMed] [Google Scholar]
- 20.Law, M., G. C. Carter, K. L. Roberts, M. Hollinshead, and G. L. Smith. 2006. Ligand-induced and non-fusogenic dissolution of a viral membrane. Proc. Natl. Acad. Sci. USA 103:5989-5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, C. L., C. S. Chung, H. G. Heine, and W. Chang. 2000. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 74:3353-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locker, J. K., A. Kuehn, S. Schleich, G. Rutter, H. Hohenberg, R. Wepf, and G. Griffiths. 2000. Entry of the two infectious forms of vaccinia virus at the plasma membrane is signaling-dependent for the IMV but not the EEV. Mol. Biol. Cell 11:2497-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maxfield, F. R. 1982. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J. Cell Biol. 95:676-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moss, B. 2005. Poxvirus entry and membrane fusion. Virology 244:48-54. [DOI] [PubMed] [Google Scholar]
- 25.Moss, B., and B. M. Ward. 2001. High-speed mass transit for poxviruses on microtubules. Nat. Cell Biol. 3:e245-e246. [DOI] [PubMed] [Google Scholar]
- 26.Ojeda, S., T. G. Senkevich, and B. Moss. 2006. Entry of vaccinia virus and cell-cell fusion require a highly conserved cysteine-rich membrane protein encoded by the A16L gene. J. Virol. 80:51-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payne, L. G. 1979. Identification of the vaccinia hemagglutinin polypeptide from a cell system yielding large amounts of extracellular enveloped virus. J. Virol. 31:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payne, L. G., and E. Norrby. 1978. Adsorption and penetration of enveloped and naked vaccinia virus particles. J. Virol. 27:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez, J. F., and G. L. Smith. 1990. Inducible gene expression from vaccinia virus. Virology 177:239-250. [DOI] [PubMed] [Google Scholar]
- 30.Senkevich, T. G., and B. Moss. 2005. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion. J. Virol. 79:4744-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senkevich, T. G., S. Ojeda, A. Townsley, G. E. Nelson, and B. Moss. 2005. Poxvirus multiprotein entry-fusion complex. Proc. Natl. Acad. Sci. USA 102:18572-18577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senkevich, T. G., B. M. Ward, and B. Moss. 2004. Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene. J. Virol. 78:2357-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 34.Townsley, A. C., T. G. Senkevich, and B. Moss. 2005. The product of the vaccinia virus L5R gene is a fourth membrane protein encoded by all poxviruses that is required for cell entry and cell-cell fusion. J. Virol. 79:10988-10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Townsley, A. C., T. G. Senkevich, and B. Moss. 2005. Vaccinia virus A21 virion membrane protein is required for cell entry and fusion. J. Virol. 79:9458-9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanderplasschen, A., M. Hollinshead, and G. L. Smith. 1998. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J. Gen. Virol. 79:877-887. [DOI] [PubMed] [Google Scholar]
- 37.Vincent, M. J., E. Bergeron, S. Benjannet, B. R. Erickson, P. E. Rollin, T. G. Ksiazek, N. G. Seidah, and S. T. Nichol. 2005. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward, B. M. 2005. Visualization and characterization of the intracellular movement of vaccinia virus intracellular mature virions. J. Virol. 79:4755-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White, J., K. Matlin, and A. Helenius. 1981. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J. Cell Biol. 89:674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]