Abstract
Interaction of simian virus 40 (SV40) major capsid protein Vp1 with the minor capsid proteins Vp2 and Vp3 is an integral aspect of the SV40 architecture. Two Vp3 sequence elements mediate Vp1 pentamer binding in vitro, Vp3 residues 155 to 190, or D1, and Vp3 residues 222 to 234, or D2. Of the two, D1 but not D2 was necessary and sufficient to direct the interaction with Vp1 in vivo. Rational mutagenesis of Vp3 residues (Phe157, Ile158, Pro164, Gly165, Gly166, Leu177, and Leu181) or Vp1 residues (Val243 and Leu245), based on a structural model of the SV40 Vp1 pentamer complexed with Vp3 D1, was carried out to disrupt the interaction between Vp1 and Vp3 and to study the consequences of these mutations for viral viability. Altering these residues to bulky, charged residues blocked the interaction in vitro. When these alterations were introduced into the viral genome, they reduced viral viability. Mutants with alterations in Vp1 Val243, Leu245, or both to glutamate were nearly nonviable, whereas those with Vp3 alterations reduced, but did not eliminate, viability. Our results defined the residues of Vp1 and the minor capsid proteins that are essential for both the interaction of the capsid proteins and viral viability in permissive cells.
Simian virus 40 (SV40), of the family Polyomaviridae, is a nonenveloped small DNA tumor virus, about 45 to 50 nm in diameter, whose capsid structure is known at atomic resolution (21, 30). Its capsid is built from pentamers of the major capsid protein Vp1 and the minor capsid proteins Vp2 and Vp3. The two minor capsid proteins are identical except that Vp2 has an amino-terminal segment of 118 residues in addition to the 234 shared carboxy-terminal residues that constitute Vp3. The unique amino terminus of Vp2 is myristoylated, a lipid modification that has been proposed to play a role in entry and uncoating during infection (16, 27).
Three sequence elements within the shared carboxy-terminal sequence have been identified and are here referred to as amino acids of Vp3. The first of these elements, residues 199 to 206, is the monopartite Vp3 nuclear localization signal (NLS), which functions in the nuclear import of newly synthesized Vp3 and in the nuclear entry of infecting particles (5, 25). This NLS interacts with the most abundant cellular NLS receptor complex, the importin α2/β1 heterodimer (25). The second element, residues 222 to 234, or D2, binds DNA (4) and is an in vitro Vp1-interactive determinant (9). Interestingly, Vp3 from the highly related murine polyomavirus (Py) lacks this Vp1-interacting determinant and DNA binding D2 and binds Vp1 via a more internal region (Py Vp3 residues 140 to 181) (1). This internal region is highly conserved among polyomaviruses. Since the crystal structure of this internal region of Py Vp3 bound to the Py Vp1 pentamer is known (3), we have built a homology model of the SV40 Vp3 fragment (Vp3 residues 157 to 184, harboring most of the 01 residues) bound to its Vp1 pentamer (15). Whether these two sequence elements, D1 and D2, function in the in vivo Vp1-Vp3 interaction is not known.
Observations reported to date imply that Vp1 and Vp3 associate soon after their synthesis in the cytoplasm. First, although both capsid proteins harbor resident NLSs (5, 13), an NLS-defective SV40 Vp3 can localize in the nucleus if wild-type Vp1 with a functional NLS is coexpressed in the same cell (13), suggesting that Vp1 and Vp3 form a complex in the cytoplasm prior to nuclear entry. The nuclear localization of Py Vp3 is promoted by coexpression of Vp1 (8, 24, 28). Second, many SV40 temperature-sensitive mutant (ts) Vp1s remain cytoplasmic, along with the coexpressed wild-type Vp3, at the nonpermissive temperature (14). Similarly, mutant Vp1s with functional Vp1-NLSs that presumably are defective in folding remain in the cytoplasm and block the nuclear entry of wild-type Vp3 (17). Finally, regardless of the amount of capsid protein synthesized in the cytoplasm, a constant ratio of Vp1 and Vp3 that reflects the ratio in the mature virion is found in the nucleus (22), implying that, by formation of the complex, the stoichiometry of Vp1 and Vp3 in the nucleus is predetermined in the cytoplasm. Assuming that these capsid proteins interact in the cytoplasm prior to nuclear import and virion assembly, it should be feasible to identify the amino acids that direct the Vp1-Vp3 interaction. Thus, using our structural model of the D1 SV40 Vp3 fragment bound to its Vp1 pentamer, we have predicted additional interactive residues for Vp1 and Vp3. Subsequent mutagenesis of these residues shows that of the two Vp3 sequence elements, D1 (Vp3 residues 155 to 190) and D2 (Vp3 residues 222 to 234), D1 is essential for the binding of Vp1 to Vp3 in the cytoplasm, and mutations within this region affect viral viability substantially, being essential for both viral infection and propagation.
MATERIALS AND METHODS
Construction of plasmids.
The amino acids common to both Vp2 and Vp3 are numbered according to the Vp3 amino acid sequence. Mutations were confirmed by DNA sequencing. The sequences of oligonucleotide linkers or PCR primers used in this study are available upon request.
A series of pQE16-based plasmids was constructed for expression of polyhistidine-tagged fusion proteins of dihydrofolate reductase (DHFR) and Vp3 fragments (DHFR-Vp3 open reading frames [ORFs]) in Escherichia coli. pQE-Vp33-234, pQE-Vp3165-234, pQE-Vp3195-234, pQE-Vp3165-206, and pQE-Vp3222-234 have been described previously (24). pQE-Vp3155-190 was made by inserting a PCR fragment encoding Vp3 residues 155 to 190 followed by a stop codon into pQE-Vp3195-234 through the NotI and XhoI sites. pQE-Vp3ΔD1 was made by replacing the PstI-to-RsrII fragment of pQE-Vp33-234 with an analogous PCR fragment that lacks Vp3 residues 155 to 190. pQE-Vp33-221 and pQE-Vp3ΔD1/2 were made by introducing a stop codon after the 222nd residues of pQE-Vp33-234 and pQE-Vp3ΔD1, respectively, to delete the D2 region.
A series of pBS-based Vp3 plasmids was created for synthesis of Vp3-derivative proteins by in vitro transcription and translation from the T7 promoter. pBSVp3(euk) was made from pBS3-234 (25) by inserting a linker encoding the first three amino acids of Vp3 through the NotI and SalI sites and by replacing the XmaI-to-XhoI region with an analogous PCR fragment generated from nonoverlapping SV40 plasmid (NO-pSV40) SRBSM (25). pBSVp3Δ6-53 and pBSVp3Δ6-94 were made by replacing the SalI-to-AvrII fragment and SalI-to-PstI fragment of pBSVp3(euk), respectively, with a linker that deletes the coding region for Vp3 amino acids 6 to 53 and 6 to 94, respectively.
A series of Vp1 point mutations, Vp1 Val 243 to Glu (V243E), Leu245 to Glu (L245E), and Val243-L245 to Glu (V243E-L245E), was made in pBS-Vp1 by PCR-based overlap extension (12). Briefly, two PCR fragments were generated using two pairs of primers: SV40 S1909-1928 (5′-AAACTCATGAAAATGGTGCT-3′) and its antisense counterpart to amplify the mutation-containing region together with upstream sequences and SV40 AS2334-2359 (5′-TCTGGGAAGTCCCTTCCACTGCTGTG-3′) and its antisense counterpart to amplify the mutation-containing region together with downstream sequences, encompassing the Vp1 coding region from Lys134 to Arg284. The two overlapping PCR products were mixed and used as a template to generate PCR fragments encompassing Vp1 amino acids 161 to 252, and the resulting fragments were inserted into pBS-Vp1 (20) via the PstI and ApaI sites.
A series of pSG5-based plasmids was also created for synthesis of Vp3-derivative proteins in vitro. pSGVp3 was described previously (13). pSGVp3(euk) was made by inserting via the NotI and BglII sites the full-length Vp3 fragment of pBSVp3(euk) into a pSG5 derivative, namely, pSG5-NBX, which harbors the NotI, BamHI, and XhoI sites between the EcoRI and BglII sites of pSG5, allowing eukaryotic expression and in vitro translation of Vp3 from the dual SV40 early and T7 promoters. pSGVp31-221, pSGVp31-199, and pSGVp31-166 were generated by inserting a PCR fragment into pSGVp3(euk) via the RsrII and BglII sites that introduces a stop codon at the 222nd, 200th, and 167th Vp3 residues, respectively. pSGVp3ΔD1 (deleted of Vp3 amino acids 155 to 190) was constructed by sequentially ligating three fragments, the 389-bp PflMI-to-RsrII fragment of pQE-Vp3ΔD1, the 120-bp RsrII-to-BglII fragment of pBSVp3(euk), and the 4.3-kbp BglII-to-PflMI fragment of pSGVp31-199. pSGVp3NLS(-)ΔD1 and pSGVp3NLS(-)ΔD1/2 were made by replacing the RsrII-to-BsrG1 fragment of pSGVp3ΔD1, encoding the Vp3 NLS and D2 region, with a linker in which the NLS was altered (KKKRK to NNNGN) or D2 was deleted in addition to the NLS alteration. pSGVp3NLS(-) was made by replacing the Vp3 amino acid 200 to 234 coding region of pSGVp3(euk) with the analogous PCR fragment generated from pSV-Vp3Null NG (25).
Vp3 point mutations Phe157-Ile158 to Glu (F157E-I158E), Pro164-Gly165-Gly166 to Arg-Glu-Arg (P164R-G165E-G166R) or Glu-Arg-Glu (P164E-G165R-G166E), Leu177 to Glu (L177E), Leu181 to Glu (L181E), Leu177-Leu181 to Glu (L177E-L181E), and Vp3 Leu179-Leu183 to Glu (L179E-L183E) or Ala (L179A-L183A) were introduced into pSG5Vp3(euk) via overlap extension as described above for the construction of pBS-Vp1 point mutants. Using pBS-Vp1 as the template, one pair of primers, PL1 (5′-GGTATA ACT CTCTGCAGGATTACTACTCTAC-3′) and its antisense counterpart, was used to amplify the mutation-containing region together with upstream sequences, and a second pair, AS 1766-1792 (5′-CAA AGG AAT TCTAGC CAC CTG TAG CA-3′) and its antisense counterpart, was used to amplify the mutation-containing region and its downstream region. The two PCR fragments were mixed and served as the template to generate a longer PCR fragment encoding Vp3 amino acids 95 to 200, using PL1 and AS 1766-1792 as the primers. The resultant fragment was inserted into pSG5Vp3(euk) via the PstI and RsrII sites to make plasmids harboring corresponding mutations.
Mutagenesis to disrupt Vp1-Vp3 interaction was planned based on a model of the SV40 Vp1 pentamer bound to the Vp3 D1 fragment (see Fig. 4).
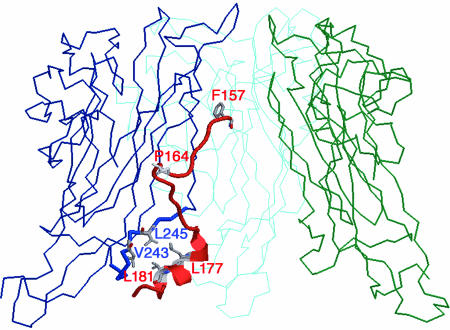
FIG. 4.
Structural model of an SV40 Vp1 pentamer-D1 Vp3 fragment complex. A side view of a structural model of a complex composed of the SV40 Vp1 pentamer and the Vp3 fragment (red), Vp3 residues 157 to 184 (15), is shown. To simplify the view, three out of five Vp1 subunits of the pentamer, colored blue, cyan, and green, are shown. Vp1 residues from amino acids 50 to 300 are shown. Residues of Vp1 and Vp3 that involve the interaction are highlighted in gray.
For the construction of mutant viral genomes, mutations introduced into a coding region of either Vp1 in pBS-Vp1s or Vp3 in pSG5Vp3s, described above, were transferred to NO-pSV40 SRBSM, which carries a fully viable viral genome (25). In brief, the AflII-to-ApaI fragments of the respective mutant pBS-Vp1s were exchanged with the corresponding fragment of NO-pSV40 SRBSM to make NO-pSV40 Vp1 V243E, NO-pSV40 Vp1 L245E, and NO-pSV40 Vp1 V243E-L245E. The Vp3 mutants were made by replacing the SalI-to-RsrII region of NO-pSV40 SRBSM with the corresponding fragments from mutant pSGVp3s to generate a series of NO-pSV40 Vp3 mutants, F157E-I158E, P164R-G165E-G166R, P164E-G165R-G166E, L177E, L181E, L177E-L181E, L179E-L183E, and L179A-L183A. NO-pSV40 Vp3ΔC13 was made by replacing the RsrII-to-XbaI region with a linker containing a stop codon after the 221st codon of Vp3. Prior to use in transfection experiments, NO-pSV40 plasmids were digested with BamHI and recircularized to yield NO-SV40 viral genomes as described previously (13).
Expression, purification, and iodination of recombinant proteins and in vitro transcription/translation.
The expression and purification of glutathione S-transferase (GST), GST-Vp3, and Vp1ΔC58 (16) and of DHFR and the DHFR-Vp3 ORF series (25) have been described previously. The purified proteins were quantified by Coomassie blue staining following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and by use of a bicinchoninic acid protein quantification kit (Pierce).
DHFR and DHFR-Vp3 ORFs were iodinated to a specific activity of 8,000 to 12,000 cpm/fmol by use of Iodobeads (Pierce), diluted to a 10-ng/μl concentration in binding buffer (see Materials and Methods for in vitro interaction assay), and clarified by centrifugation at 14,000 × g for 10 min. The soluble protein concentration was estimated by SDS-PAGE followed by phosphorimaging.
[35S]methionine-labeled Vp3 derivatives were synthesized in vitro from pBSVp3 or pSGVP3 plasmid series as templates by use of the TNT quick coupled transcription/translation system (Promega). 35S-labeled Vp1 derivatives were similarly synthesized from pBS-Vp1s. The specific activities of the 35S-labeled proteins were determined by measuring trichloroacetic acid precipitable counts per μg protein.
In vitro binding assay.
The interaction of Vp3 with Vp1 was tested by binding of radiolabeled test protein Vp1 or Vp3 to nonradiolabeled Vp3 or Vp1, respectively. The nonradiolabeled protein contains an affinity tag and can be isolated via affinity chromatography in the following manner. First, approximately 10 fmol of 35S-labeled wild-type or mutant Vp3s and 900 fmol of purified Vp1ΔC58(His) pentamer were mixed in 100 μl of binding buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 0.1% Triton X-100, 0.1% sodium deoxycholate, 1 mM MgCl2, 2 mM β-mercaptoethanol, 50 ng/ml ethidium bromide, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml pepstatin) and incubated for 30 min at room temperature. Then, 10 μl of Talon beads (Clontech) in 400 μl of binding buffer was added and the mixture was rocked for 30 min. In the second binding assay, approximately 10 fmol of 125I-labeled DHFR-Vp3s and 100 fmol of Vp1ΔC58 pentamer were mixed in 100 μl binding buffer and incubated for 2 h at 4°C. Fifteen microliters of protein A-Sepharose (Amersham Pharmacia), cross-linked with affinity-purified anti-Vp1 immunoglobulin Gs by use of dimethyl pimelimidate (Pierce) and pretreated with 5% skim milk blocking agent in phosphate-buffered saline (PBS) for 10 min followed by several washes in PBS, was brought to 300 μl with binding buffer and added to the Vp1-Vp3 binding assay mixtures, which were further incubated for 2 h. For both types of assay, the resulting beads were extensively washed with binding buffer, and bead-bound proteins were eluted and denatured in SDS-sample buffer and analyzed by SDS-PAGE followed by phosphorimaging. For the third type of binding assay, glutathione-Sepharose beads (Amersham Pharmacia) bound with GST or GST-Vp3 were mixed with 35S-labeled Vp1s in its in vitro translation lysate, incubated, washed extensively, and analyzed for tightly bound proteins by use of SDS-PAGE followed by fluorography as previously described (19).
Cells, microinjection, transfection, immunocytochemistry, and plaque assay.
The conditions for TC-7 and CV-1 cell cultures, microinjection, and immunocytochemistry to detect the expression and subcellular localization of Vp1 or Vp3 have been described previously (25). For microinjection, at least 100 TC7 cell nuclei in the same area of the coverslip were injected with DNA solution in PBS, with either 20 pM pSGVp3s alone or 20 pM each of pSGVp3s and pSGVp1 (13), and fixed 6 h postinjection.
CV-1 cells transfected with either wild-type or mutant NO-SV40 viral DNA were lysed by sonication at 72 h posttransfection, and the lysates, adjusted to 0.5 μg/ml of viral DNA, were used for plaque formation assays via infection of CV-1 cells as described previously (20, 25). Note that the quantity of plaques described in Table 1 was determined from 2 ml of the lysate. The extent of viral DNA replication was examined by isolating episomal DNA from 1/10 of the transfected cells from one 150-mm dish by the Hirt method, digestion with DpnI, and quantitation by Southern blotting as described previously (19). The relative amounts of capsid proteins produced were determined by analyzing 1/200 (for Vp1) or 1/50 (for Vp3) of the transfected cells from one 150-mm dish by Western blotting.
TABLE 1.
Viabilities of mutants defective in Vp1-Vp3 interactiona
| NO-SV40 | Viability |
|---|---|
| Wild type | 1.2 × 108 |
| Vp3 F157E-I158E | 1.7 × 105 |
| Vp3 P164R-G165E-G166R | 3.2 × 105 |
| Vp3 P164E-G165R-G166E | 1.8 × 105 |
| Vp3 ΔC13 | 2.0 × 107 |
| Vp1 V243E | <1 |
| Vp1 L245E | 46 |
| Vp1 V243E- L245E | <1 |
Viability was determined as described previously (25) and is based on the number of plaques formed by infection with viral lysate containing 1 μg of replicated viral DNA.
A homology model of the SV40 Vp1 pentamer-Vp3 D1 complex.
A homology model of an SV40 Vp1 pentamer-Vp3 D1 complex has been built based on the crystal structure of the Py Vp1-Vp3 complex (3) and has been described previously (15). The structures of SV40 and polyomavirus pentamers are well conserved (21, 30, 31). Based on these structures (PDB codes 1CN3 and 1SVA), we built a homology model in the following way. The polyomavirus complex was superimposed onto the Vp1 pentamer of SV40 by overlaying the Cα positions of the β-sheets of the two Vp1s (the backbones can be superimposed with a root mean square deviation of ∼0.5 Å). The ordered region of Vp3 observed in the polyoma virus structure (residues 154 to 181) is highly conserved within SV40 Vp3 (residues 157 to 184), and the polyoma virus Vp3 side chains with library torsion angles were replaced with their SV40 counterparts by use of TURBO-FRODO. Residues Trp 175 through Tyr 184 form a hydrophobic α-helix at the base of the SV40 Vp1 pentamer. SV40 Vp3 residues Leu 177 and Leu 181 make hydrophobic contacts with Vp1 residues Val 243 and Leu 245, respectively. The SV40 Vp1-Vp3 complex was built and inspected using TURBO-FRODO (http://afmb.cnrs-mrs.fr/rubrique113.html). Figure 4 was made using RasMOL (http://www.umass.edu/microbio/rasmol/index.html).
RESULTS
Two Vp3 sequence elements are involved in Vp1 binding in vitro.
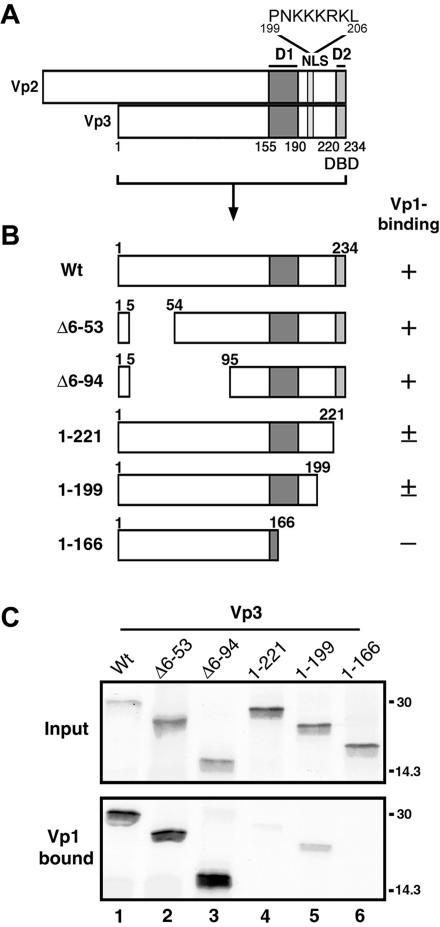
In order to identify the Vp3 sequences that are involved in Vp1 binding, we first constructed a series of truncated Vp3s (Fig. 1B) and tested them for binding to the Vp1 pentamer in vitro (Fig. 1C). The locations for the Vp3-NLS and the putative Vp1 interactive elements, D1 and D2, are marked in Fig. 1A. The recombinant Vp1 pentamer, Vp1ΔC58, is the one in which the 58 carboxyl residues are replaced with a polyhistidine tag and Cys 104 and 254 have been replaced with alanines to eliminate the potential aggregation of the Vp1 pentamer (19). The abilities of 35S-labeled wild-type and mutant Vp3s to bind to the Vp1ΔC58 pentamer were tested by capturing the radiolabeled proteins bound to Vp1 on metal chelate beads (Fig. 1C, lower panel). In the upper panel of Fig. 1C, the amount of labeled protein used in each reaction is shown. Wild-type Vp3 bound fully to the Vp1 pentamer (Fig. 1C, lane 1), and nearly 100% of the input Vp3s were retrieved bound to Vp1. Deleting Vp3 amino-terminal residues 6 to 53 or 6 to 94 did not affect Vp1 binding (Fig. 1C, lanes 2 and 3). Deleting D2 (Fig. 1C, lane 4) or the last 35 residues of Vp3, which include the NLS and D2 (Fig. 1C, lane 5), reduced, but did not eliminate, Vp1 binding; 13% and 20% of the input proteins bound to Vp1, respectively. No Vp1 binding was detected when the last 68 residues, comprising most of D1 and all of D2, of Vp3 were deleted (Fig. 1C, lane 6). Together, these results show that both Vp3 sequence elements, D1 and D2, participate in Vp1 binding in vitro.
FIG. 1.
In vitro interaction of truncated Vp3s and the Vp1 pentamer. (A) Functional Vp3 sequence elements. Minor capsid proteins Vp2 and Vp3 share identical amino acids, except for a Vp2-unique segment of 118 residues; hence, the shared regions are denoted by Vp3 amino acids as follows: D1, Vp3 residues 155 to 190; D2, Vp3 residues 222 to 234; and NLS, Vp3 residues 199 to 206 (whose sequence is shown). (B) Schematic diagram of truncated Vp3s used in the Vp1 pentamer binding assay. The first and last Vp3 residue numbers in the fragments are shown in each diagram. The abilities of the Vp3 fragments to bind to Vp1ΔC58 as shown in Fig. 1C are categorized by +, ±, and −, describing strong, weak, and undetectable, respectively. (C) Vp1ΔC58 pentamer was incubated with 35S-methionine labeled wild-type or truncated Vp3s and retrieved by metal-chelate beads. The Vp3s bound to the beads were visualized by SDS-PAGE and autoradiography. The upper panel (Input) and lower panel (Vp1 bound) show 1/25 of the total 35S-labeled Vp3 used and 1/3 of the total 35S-labeled Vp3 bound to the beads, respectively. Positions for the molecular-mass marker, 30 and 14.3 kDa, are marked on the right side of the panels. Vp3 ORFs used for binding assays are as follows: lane 1, full-length Vp3 (Wt); lane 2, Δ6-53; lane 3, Δ6-94; lane 4, residues 1 to 221; lane 5, residues 1 to 199; and lane 6, residues 1 to 166.
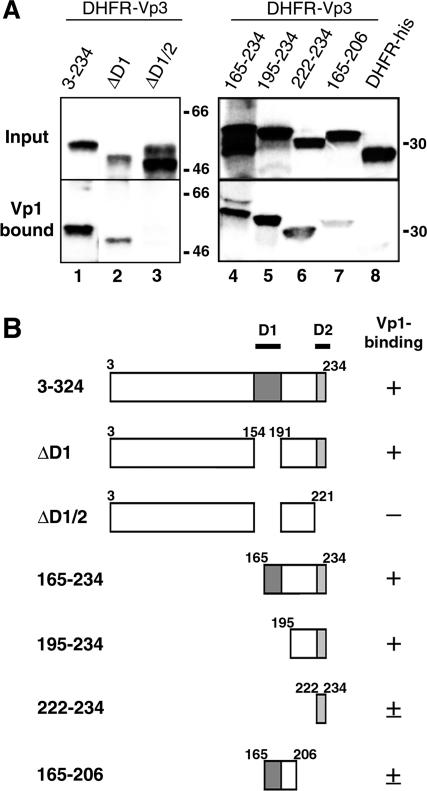
We next tested whether Vp3 D1 and D2 are sufficient for Vp1 binding. A series of 125I-labeled DHFR fusion proteins harboring Vp3 ORFs (Fig. 2B) was tested for the ability to bind to the unlabeled Vp1ΔC58 pentamer. Labeled Vp3s that bound Vp1 were immunoprecipitated using protein A beads cross-linked with affinity-purified anti-Vp1 antibody, resolved with SDS-PAGE, and subjected to autoradiography. Nearly full-length Vp3 (Vp33-234) (Fig. 2A, lane 1) and the truncated Vp3 containing the last 70 residues (Vp3165-234) (Fig. 2A, lane 4) bound fully to Vp1. This is expected since Vp3165-234 encompasses two-thirds of the D1 and the entire D2 sequence element. Removing the D1 sequence either via an internal deletion of the nearly full-length Vp3 (Fig. 2A, lane 2) or via deletion of 194 amino-terminal residues, leaving the last 40 residues of Vp (Vp3195-234) (Fig. 2A, lane 5), did not affect Vp1 binding. Having D2 alone in the context of the DHFR fusion protein was sufficient for binding of Vp1 (residues 222 to 234) (Fig. 2A, lane 6). The fusion protein having only two-thirds of D1 showed low Vp1 binding (residues 165 to 206) (Fig. 2A, lane 7). As expected, the protein either lacking both D1 and D2 (ΔD1/2) (Fig. 2A, lane 3) or having no Vp3 fragment (DHFR-His) (Fig. 2A, lane 8) exhibited little Vp1 binding. Thus, D1 and D2 are sufficient for Vp1 binding in vitro.
FIG. 2.
In vitro interaction of DHFR-Vp3 fragments and the Vp1 pentamer. (A) Recombinant DHFR-Vp3 ORFs and DHFR-His labeled with 125I were incubated with Vp1ΔC58 and immunoprecipitated with anti-Vp1 antibody. The precipitates were resolved with SDS-PAGE, and radioactive protein bands of DHFR fusion proteins were visualized by autoradiography. The upper panel shows 1/10 of the amount of protein used for each reaction (Input), and the lower panel shows 1/2 of the amount of the Vp1ΔC58-reacted proteins (Vp1 bound). Positions for the molecular-weight marker are marked on the right side of the panel. The following Vp3 fragments fused with DHFR protein were used: lane 1, residues 3 to 234; lane 2, 3-234Δ155-190 (ΔD1); lane 3, 3-234Δ155-190 and Δ222-234 (ΔD1/2); lane 4, residues 165 to 234; lane 5, residues 195 to 234; lane 6, residues 222 to 234; lane 7, residues 165 to 206; and lane 8, unfused DHFR-His. (B) Schematic diagram of Vp3 fragment fused to histidine-tagged DHFR and the extent of Vp1 binding. Numbers and symbols are defined in the legend to Fig. 1B.
Vp3 D1 but not D2 mediates Vp1 binding in vivo.
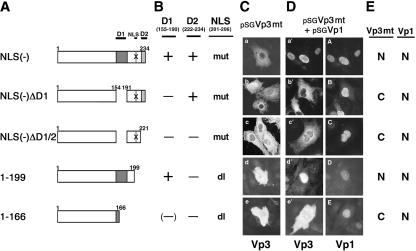
We have shown that an NLS-defective Vp3 can enter the nucleus when an NLS-intact wild-type Vp1 is expressed in the same host cell cytoplasm (13). Such functional NLS complementation, which occurs at the protein level as long as both proteins interact, can be used to determine whether either of the two Vp3 sequence elements functions in Vp1 interaction in vivo. A series of pSGVp3 plasmids was made to allow for the expression of wild-type and various mutant Vp3s, which contain either a deletion or point mutations in Vp3-NLS in conjunction with a deletion of D1, D2, or both (pSG Vp3s) (Fig. 3A and B). The plasmids expressing mutant Vp3s were microinjected with or without a Vp1 expression plasmid, pSGVp1, into the nuclei of TC-7 cells. A cytoplasmic Vp1-Vp3 interaction is evident when the NLS-defective Vp3 colocalizes with Vp1 in the nucleus. NLS-defective mutant Vp3s without a functional Vp1-interactive domain remain in the cytoplasm.
FIG. 3.
Vp3 D1 directs cytoplasmic Vp1 interaction. TC-7 cells grown on coverslips were injected with the respective pSGVp3 constructs with or without pSGVp1. (A) Schematic diagrams of pSGVp3 constructs in which regions are deleted from the mutant Vp3s are shown. X indicates mutations of Vp3 NLS 201-KKKRK-205 to NNNGN. (B) Locations of Vp3 mutations and deletions. mut, mutation; dl, deletion. Other abbreviations are defined in the legend to Fig. 1B. (C) Subcellular localization of mutant Vp3s following microinjection of the respective pSGVp3 constructs. (D) Each set of photographs shows subcellular localization of Vp3 on the left and Vp1 on the right, in the cells injected with a mixture of the respective pSGVp3 and pSGVp1 mutants. (E) Summary of the subcellular localization of Vp3 mutants and coexpressed Vp1. N, nuclear localization; C, cytoplasmic localization.
All NLS-defective Vp3s expressed without Vp1 remained in the cytoplasm (Fig. 3C). When coexpressed with wild-type Vp1, the NLS-defective Vp3 with intact D1 and D2 localized to the nucleus [NLS(-)] (Fig. 3D, panels a′ and A). The localization of Vp31-199, lacking D2 and the resident NLS, was mostly in the nucleus along with the coexpressed Vp1 (residues 1 to 199) (Fig. 3D, panels d′ and D). This result shows that a Vp3 missing D2 still binds to Vp1 in vivo. In contrast, the NLS-defective Vp3 missing D1 remained in the cytoplasm and failed to colocalize in the nucleus with Vp1 [NLS(-)ΔD1] (Fig. 3D, panels b′ and B). Thus, it is D1 that mediates the binding to Vp1 in the host cell cytoplasm. Not surprisingly, Vp3 lacking both D1 and D2, either through an internal deletion of D1 and truncation of D2 along with the defective NLS [NLS(-)ΔD1/D2] (Fig. 3D, panels c′ and C) or through a carboxyl terminus truncation that removes D2, NLS, and two-thirds of D1 (residues 1 to 166) (Fig. 3D, panels e′ and E), did not colocalize with Vp1 in the nucleus. A summary indicating the presence or absence of subcellular colocalization of mutant Vp3s with Vp1 is shown in Fig. 3E. We conclude that Vp3 D1 is essential for Vp1 interaction in vivo, whereas D2 is not.
Structure-based mutagenesis of Vp3 D1 defines residues critical for binding to Vp1 in vitro.
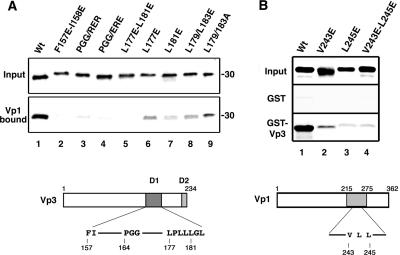
The mutagenesis to disrupt the Vp1-Vp3 D1 interaction was aimed at regions within the Vp3 revealed in the model of the SV40 Vp1-Vp3 complex (Fig. 4) (15). The first ordered residue of Vp3 is Phe157, which, together with Ile158, nestles into the hydrophobic neck at the top of the conical interior of the Vp1 pentamer. Alteration of Phe157 and Ile158 to glutamate is predicted to disrupt contact with Vp1 Pro231 as well as to disrupt proper positioning of the upstream Vp3 amino acids. Second, residues Pro164, Gly165, and Gly166 form a tight turn, where they pack into a crevice between the two main β-sheets of Vp1. In addition, Pro164 and Gly165 have unusual main chain torsion angles, leading to both steric and conformational restraints. Their alteration to charged residues is also predicted to disrupt Vp1-Vp3 interaction. Third, residues Trp175 through Tyr184 form a hydrophobic α-helix at the base of the Vp1 pentamer; Vp3 residues Leu177 and Leu181 make hydrophobic contacts with Vp1 residues Val243 and Leu245, respectively. Thus, we predicted that mutating Vp3 residues Leu177 and Leu181 to glutamate or Vp1 residues Val243 and Leu245 to glutamate would disrupt Vp3-Vp1 interaction. For comparison, Vp3 Leu179 and Leu183, whose side chains point away from the Vp1 pentamer, were mutated to a charged residue or a neutral residue, glutamate or alanine.
Based on these predictions, Vp3 mutants Vp3 Phe157 and Ile158 to Glu (F157E-I158E), Vp3 Pro164-Gly165-Gly166 to Arg-Glu-Arg (P164R-G165E-G166R) or Glu-Arg-Glu (P164E-G165R-G166E), Vp3 Leu177 and Leu181 to Glu (L177E-L181E), and Vp3 Leu179 and Leu183 to Glu (L179E-L183E) or Ala (L179A-L183A) were made. The extent of the binding of input 35S-labeled mutant Vp3s (Fig. 5A, upper panel) to the Vp1ΔC58 pentamer (Fig. 5A, lower panel) was tested in vitro. Wild-type Vp3 fully bound to Vp1ΔC58 (Fig. 5A, lane 1). Mutant Vp3s F157E-I158E (Fig. 5A, lane 2), P164R-G165E-G166R (Fig. 5A, lane 3), P164E-G165R-G166E (Fig. 5A, lane 4), and L177E-L181E (Fig. 5A, lane 5) showed reduced Vp1 binding, from 13% to 20% of wild-type binding levels. The Vp1 binding of the single-point mutant Vp3s, Leu177 to Glu (L177E) and Leu181 to Glu (L181E), was also reduced (57% [Fig. 5A, lane 6] and 33% [Fig. 5A, lane 7], respectively). Mutagenesis of Leu179 and Leu183 to glutamate or alanine did not reduce Vp1 binding as much as the Leu177 and Leu181 counterparts (Fig. 5A, compare lanes 8 and 9 with lane 5). We note that the single Vp3 point mutation was sufficient to reduce Vp1 binding (Fig. 5A, lanes 2 to 5). This result is in contrast with the Vp1 binding observed for DHFR fusion Vp3 missing the D1 segment (Fig. 2A, lane 2). The significant residual Vp1 binding detected for DHFR-Vp3 ΔD1 probably reflects the presence of the D2 segment, which also confers Vp1 binding, in the fusion protein (Fig. 2A, lanes 3, 5, and 6). The simplest interpretation is that D2 assumes a different local structure in the fusion protein than in the context of Vp3, leading to the observed difference in intensity of Vp1 binding.
FIG. 5.
In vitro interaction of point mutant Vp1s and Vp3s. (A) 35S-labeled Vp3s were reacted with the Vp1ΔC58 pentamer, and the bound proteins were separated by SDS-PAGE followed by fluorography. The upper panel shows one-fifth of the Vp3 protein used for the reaction (Input). The lower panel shows one-half of the protein retrieved from the Vp1ΔC58-bound beads (Vp1 bound). A schematic diagram of Vp3 with locations for mutations within D1 marked is shown at the bottom. The wild type (Wt) and the following mutant Vp3 proteins were examined: lane 1, wild type; lane 2, F157E-158E; lane 3, PGG/RER; lane 4, PGG/ERE; lane 5, L177E-L181E; lane 6, L177E; lane 7, L181E; lane 8, L179E/L183E; and lane 9, L179A/L183A. (B) 35S-labeled Vp1s were reacted with either GST or GST-Vp3, and the bound proteins were retrieved by glutathione-Sepharose beads. The bound Vp1 was visualized by SDS-PAGE followed by fluorography. The upper panel shows one-fifth of the input count used for reaction (Input), the middle panel shows one-half of the GST-bound Vp1s (GST), and the bottom panel shows one-half of the GST-Vp3-bound Vp1s (GST-VP3). Compared to what was found for the wild type, 53%, 14%, and 9% of the Vp1 V243E, L245E, and V243E-L245E mutants, respectively, were retrieved from the GST-Vp3-bound beads. A schematic diagram of Vp1 with mutations marked is shown at the bottom. The following 35S-labeled Vp1s were used: lane 1, wild type; lane 2, V243E; lane 3, L245E; and lane 4, V243E-L245E.
Vp1 mutations to disrupt the hydrophobic contacts of Vp1 Val243 and Leu245 with Vp3 Leu177 and Leu181, respectively, were made, and the abilities of mutant Vp1s to bind to Vp3 were tested. 35S-labeled mutant Vp1s V243E, L245E, and V243E-L245E, whose input amounts are shown (Fig. 5B, upper panel), were incubated with either GST-Vp3, a recombinant Vp3 fused with glutathione S-transferase (GST-Vp3) (Fig. 5B, lower panel), or GST alone (Fig. 5B, middle panel). The proteins bound to the immobilized glutathione beads were resolved with SDS-PAGE, and the mutant Vp1s bound to GST-Vp3 were visualized by fluorography. Wild-type Vp1 bound fully to GST-Vp3 (Fig. 5B, lane 1), whereas all Vp1 mutants bound to GST-Vp3 to lesser extents (Fig. 5B, lanes 2 to 4). The fact that a substantial reduction in Vp1 binding was observed for these glutamate substitutions suggests that hydrophobic Vp1-Vp3 contacts involving Vp1 Val243 and Leu245 are disrupted.
In summary, our targeted mutagenesis of Vp1 and Vp3D1 identified amino acids that mediate their interaction in vitro. They are Vp3 residues Phe157-Ile158, Pro164-Gly165-Gly166, and Leu 177-Leu 181 and Vp1 residues Val 243 and Leu 245. The alterations of these residues to bulky, charged residues blocked or reduced interaction between Vp1 and Vp3 in vitro.
Viability of mutants.
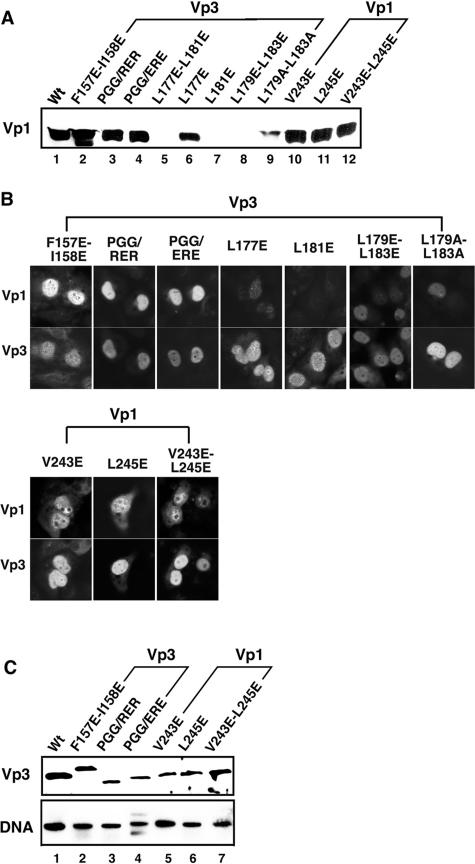
We next examined the mutants' overall viability. The point mutants described above were introduced into the viable nonoverlapping SV40 viral genome, NO-SV40 (25), and the mutants were tested for their abilities to express viral capsid proteins (Fig. 6) and their overall viability by plaque assay (Table 1). The mutant DNA was transfected into CV-1 cells and examined, first, for Vp1 expression by Western blotting (Fig. 6A, lanes 2 to 4 [Vp3 mutants] and 10 to 12 [Vp1 mutants]). The cell lysates of the Vp3 mutants, F157E-I158E (Fig. 6A, lane 2), P164R-G165E-G166R (Fig. 6A, lane 3), and P164E-G165R-G166E (Fig. 6A, lane 4), and the Vp1 mutants, V243E (Fig. 6A, lane 10), L245E (Fig. 6A, lane 11), and V243E-L245E (Fig. 6A, lane 12), contained normal levels of Vp1, equivalent to that seen in the wild-type lysate (Fig. 6A, lane 1). The subcellular localization of the mutant capsid proteins showed normal nuclear distribution (Fig. 6B). In contrast, the cells transfected with L177E (Fig. 6A, lane 6) had slightly less Vp1, and mutants L177E-L181E (Fig. 6A, lane 5), L181E (Fig. 6A, lane 7), L179E-L183E (Fig. 6A, lane 8), and L179A-L183A (Fig. 6A, lane 9) produced very little Vp1. The mutant Vp3s were found in the nucleus, though showing punctate nuclear staining to various degrees (Fig. 6B). Little wild-type Vp1 was detected and localized to the nucleus in respective mutant-transfected cells (Fig. 6B). The alterations of the Vp3 coding region in these mutants, thus, show pleiotropic effects, and the viabilities of the mutants were not examined further. The viabilities of the mutants expressing wild-type levels of both Vps (Fig. 6A, B, and C) and of DNA replication (Fig. 6C, lanes 1 to 7) were examined.
FIG. 6.
Levels of capsid proteins and viral DNA in cells transfected with mutant DNAs. (A) Level of Vp1. Lysates of the cells transfected with either wild-type or mutant NO-SV40 DNA were examined for Vp1 by immunoblotting. Approximately 1/200 of the lysates of cells transfected with wild-type DNA (lane 1), Vp3 mutants (lane 2, F157E-158E; lane 3, PGG/RER; lane 4, PGG/ERE; lane 5, L177E-L181E; lane 6, L177E; lane 7, L181E; lane 8, L179E-L183E; lane 9, L179A/L183A), and Vp1 mutants (lane 10, V243E; lane 11, L245E; lane 12, V243E-L245E) were used for immunoblotting. (B) Subcellular localization of capsid proteins. TC-7 cells grown on coverslips were transfected with each viral DNA, fixed at 24 h posttransfection, and then incubated with guinea pig anti-Vp1 and rabbit anti-Vp3 sera, followed by fluorescein- and rhodamine-conjugated secondary antibodies (22). In each pair of photos, the top and bottom panels show identical cells stained for Vp1 (top) and Vp3 (bottom). A set of photographs for cells transfected with Vp3 mutants is bracketed as Vp3, and those with Vp1 mutants are bracketed as Vp1. The cells transfected with Vp3 L177E-L181E did not show positive signs for the capsid proteins and thus are not shown. (C) Levels of Vp3 and viral DNA. The mutants showing normal levels of Vp1 in panel A were tested for level of Vp3 by Western blotting and DNA by Southern blotting. Vp3 mutants F157E-I158E (lane 2), P164R-G165E-G166R (PGG/RER) (lane 3), and P164E-G165R-G166E (PGG/ERE) (lane 4) and Vp1 mutants V243E (lane 5), L245E (lane 6), and V243E-L245E (lane 7) are marked.
The lysates of the cells transfected with these mutant DNAs were adjusted to contain 0.5 μg/ml DNA and were used to infect CV-1 cells for plaque assays (Table 1). The Vp3 mutants, F157E-I158E, P164R-G165E-G166R, and P164E-G165R-G166E, had decreased viabilities. They produced 500- to 1,000-fold fewer plaques than the wild type. All Vp1 mutants, V243E, L245E, and V243E-L245E, produced very few plaques and were nearly nonviable. Thus, we show that the mutations affecting the interaction of Vp1 with Vp3-D1 also abrogated overall viability. As a comparison, a Vp3 mutant truncated for the last 13 residues, which corresponds to the entire D2 element, was examined. The viability was largely similar to that of the wild type, reduced only fivefold (Table 1), consistent with the observation that D2 does not direct Vp1-Vp3 binding in vivo (Fig. 3).
DISCUSSION
Amino acids of SV40 capsid proteins that direct the binding of the major capsid protein Vp1 to the minor capsid proteins Vp2 and Vp3 were identified. Two Vp2 and Vp3 sequence elements, residing in the common region (here, the common region is designated Vp3), were necessary and sufficient for binding to Vp1 in vitro. They are an internal D1 element, Vp3 residues 155 to 190 (1), and a carboxy-terminal D2 element, Vp3 residues 222 to 234 (8). By contrast, D1 but not D2 mediated binding to Vp1 in vivo. NLS-less Vp3 could enter the nucleus only when NLS-intact Vp1 was expressed in the same cell. Vp3 D1 was required for this nuclear entry, while D2 was not. By targeted mutagenesis, based on a homology model of a SV40 Vp1-Vp3 D1 complex, we identified amino acids of Vp1 and Vp3 that mediate their interaction. For the Vp3 mutations that inhibited binding to Vp1, viability was reduced, while for the Vp1 mutations that eliminated binding to Vp3, viability was abolished. These results show that disruption of Vp1-Vp3 interaction leads to reduced infectivity of SV40. Further study should allow us to define distinct roles for Vp1 and Vp3 in SV40 infection.
Surprisingly, D2 was not essential in vivo as the mutant Vp3 without D2 and, without a resident Vp3-NLS, entered the nucleus piggybacked by wild-type Vp1 (Fig. 3D). In addition, it is significant that the mutant lacking D2 was quite viable (Table 1). D2 has been shown to be the DNA-binding domain (DBD) of both proteins, Vp3-DBD (6), and has been suggested to play a role in selective packaging of the viral genome during capsid formation (11). In view of our new result, that D2 was readily dispensable for infectivity, our previous conclusion that Vp3-DBD plays an essential role in infection (6) needs be reevaluated. The Vp3 mutations which were reported previously are in overlapping SV40 genomes and introduce nucleotide alterations in both genes, whereas the Vp3 mutants described here that make little Vp1 (Fig. 6), namely, Vp3 substitution mutations L177E, L181E, L179E-L183E, and L179A-L183A, are in nonoverlapping viral genomes that carry an unmodified coding sequence for wild-type Vp1. Therefore, the trans-dominant effect on Vp1 observed for these Vp3 point mutants must be due to alteration of the Vp3 coding sequence, for example, by inhibition of the splicing of the primary transcript.
Our focus in this study was aimed at identifying amino acids common to both Vp2 and Vp3 that direct the in vivo Vp1 interaction. The Vp2 amino-terminal glycine of both Py and SV40 is myristoylated (16), and this modification contributes to viral propagation (27). Yet, some mutants defective in myristoylation are viable (23). Moreover, both Py-Vp2 and Py-Vp3 bind to the Py-Vp1 pentamer in vitro to a similar extent (7), suggesting that the unique region of Vp2 has a minor role in binding to the Vp1 pentamer. The possibility that the unique region of Vp2 plays an additional role in the interaction of minor capsid proteins with Vp1 during infection, however, is not ruled out.
The interaction of Vp1 with Vp2 and Vp3 occurs soon after their synthesis in the cytoplasm (22). The formation of the complexes in the cytoplasm before nuclear entry maintains the proper stoichiometry of Vp1, Vp2, and Vp3 in the nucleus, thereby facilitating efficient virion assembly (22). On the other hand, it has been reported that Py-Vp1 alone, when expressed in insect cells, can localize into the nucleus, package cellular DNA, and form capsid (10, 24). Thus, it appears that the major capsid protein alone can direct particle formation. Whether such particles can package viral minichromosomes, however, is not known. In papillomavirus infection, the minor capsid protein L2 plays a role in genome packaging (26, 29, 32). As Vp3-NLS functions in the nuclear entry of the infecting SV40 (25), its incorporation into particles is likely key to the formation of infectious virus. As it is not known where the mutants' defect that leads to the reduction in viability lies, the viabilities of the mutants were tested by plaque assay with total cell lysates. A lack of Vp3 incorporation in the mutant particles or of particle assembly itself could account for the reduction in viability.
Conformational changes in polyomavirus capsids are expected to occur to expose signals important for productive infection—for cell binding, cell entry, and nuclear entry of the viral genome. Little is known about how individual signals become precisely exposed on the capsids. We have postulated a role for Vp1 amino acids that coordinate binding of two calcium ions per Vp1 monomer in cell entry and nuclear entry and have presented evidence for a role for the site 1 calcium-binding amino acids of the capsid in cell entry (18). The reported results imply that the structural alterations occur at the base of the Vp1 pentamer. Cavaldesi et al. have shown the structural changes in murine polyomavirus capsids following sialic acid binding (2). They have postulated that binding of the sialic acid moiety on the host cell membrane leads to the subsequent structural changes in minor capsid proteins (2). At present, how each of the three capsid proteins, Vp1, Vp2, and Vp3, functions in infection is not known. Further analyses of the mutants described here could identify separate roles for individual capsid proteins in the viral life cycle.
Acknowledgments
We thank Peggy Li for helpful discussions, Laurie Bankston and Ryan Newton for editing, Cesar Fernandez and Jason Chan for assistance in plasmid constructions, and Dorothy Shum for assistance in recombinant protein preparations.
This work was supported by public health service grant CA50574 and by a grant from the UCLA Academic Senate.
REFERENCES
- 1.Barouch, D. H., and S. C. Harrison. 1994. Interactions among the major and minor coat proteins of polyomavirus. J. Virol. 68:3982-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavaldesi, M., M. Caruso, O. Sthandier, P. Amati, and M. I. Garcia. 2004. Conformational changes of murine polyomavirus capsid proteins induced by sialic acid binding. J. Biol. Chem. 279:41573-41579. [DOI] [PubMed] [Google Scholar]
- 3.Chen, X. S., T. Stehle, and S. C. Harrison. 1998. Interaction of polyomavirus internal protein Vp2 with the major capsid protein Vp1 and implications for participation of Vp2 in viral entry. EMBO J. 17:3233-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clever, J., D. Dean, and H. Kasamatsu. 1993. Identification of a DNA binding domain in simian virus 40 capsid proteins Vp2 and Vp3. J. Biol. Chem. 268:20877-20883. [PubMed] [Google Scholar]
- 5.Clever, J., and H. Kasamatsu. 1991. Simian virus 40 Vp3 structural proteins harbor their own nuclear localization signal. Virology 181:78-90. [DOI] [PubMed] [Google Scholar]
- 6.Dean, D. A., P. P. Li, L. M. Lee, and H. Kasamatsu. 1995. Essential role of the Vp2 and Vp3 DNA-binding domain in simian virus 40 morphogenesis. J. Virol. 69:1115-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delos, S. E., T. P. Cripe, A. D. Leavitt, H. Greisman, and R. L. Garcea. 1995. Expression of the polyomavirus minor capsid proteins VP2 and VP3 in Escherichia coli: in vitro interactions with recombinant VP1 capsomeres. J. Virol. 69:7734-7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forstová, J., N. Krauzewicz, S. Wallace, A. J. Street, S. M. Dilworth, S. Beard, and B. E. Griffin. 1993. Cooperation of structural proteins during late events in the life cycle of polyomavirus. J. Virol. 67:1405-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gharakhanian, E., J. Takahashi, J. Clever, and H. Kasamatsu. 1988. In vitro assay for protein-protein interaction: carboxyl-terminal 40 residues of simian virus 40 structural protein VP3 contain a determinant for interaction with VP1. Proc. Natl. Acad. Sci. USA 85:6607-6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillock, E. T., S. Rottinghaus, D. Chang, X. Cai, S. A. Smiley, K. An, and R. A. Consigli. 1997. Polyomavirus major capsid protein VP1 is capable of packaging cellular DNA when expressed in the baculovirus system. J. Virol. 71:2857-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon-Shaag, A., O. Ben-Nun-Shaul, V. Roitman, Y. Yosef, and A. Oppenheim. 2002. Cellular transcription factor Sp1 recruits simian virus 40 capsid proteins to the viral packaging signal, ses. J. Virol. 76:5915-5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 13.Ishii, N., A. Nakanishi, M. Yamada, M. H. Macalalad, and H. Kasamatsu. 1994. Functional complementation of nuclear targeting-defective mutants of simian virus 40 structural proteins. J. Virol. 68:8209-8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasamatsu, H., and A. Nehorayan. 1979. Vp1 affects intracellular localization of Vp3 polypeptide during simian virus 40 infection. Proc. Natl. Acad. Sci. USA 76:2808-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasamatsu, H., J. Woo, A. Nakamura, P. Muller, M. J. Tevethia, and R. C. Liddington. A structural rationale for SV40 Vp1 temperature-sensitive mutants and their complementation. Protein Sci., in press. [DOI] [PMC free article] [PubMed]
- 16.Krauzewicz, N., C. H. Streuli, N. Stuart-Smith, M. D. Jones, S. Wallace, and B. E. Griffin. 1990. Myristylated polyomavirus VP2: role in the life cycle of the virus. J. Virol. 64:4414-44120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, P. P., A. Nakanishi, V. Fontanes, and H. Kasamatsu. 2005. Pairs of Vp1 cysteine residues essential for simian virus 40 infection. J. Virol. 79:3859-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, P. P., A. Naknanishi, M. A. Tran, K. Ishizu, M. Kawano, M. Phillips, H. Handa, R. C. Liddington, and H. Kasamatsu. 2003. Importance of Vp1 calcium-binding residues in assembly, cell entry, and nuclear entry of simian virus 40. J. Virol. 77:7527-7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, P. P., A. Nakanishi, D. Shum, P. C. Sun, A. M. Salazar, C. F. Fernandez, S. W. Chan, and H. Kasamatsu. 2001. Simian virus 40 Vp1 DNA-binding domain is functionally separable from the overlapping nuclear localization signal and is required for effective virion formation and full viability. J. Virol. 75:7321-7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, P. P., A. Nakanishi, M. A. Tran, A. M. Salazar, R. C. Liddington, and H. Kasamatsu. 2000. Role of simian virus 40 Vp1 cysteines in virion infectivity. J. Virol. 74:11388-11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liddington, R. C., Y. Yan, J. Moulai, R. Sahli, T. L. Benjamin, and S. C. Harrison. 1991. Structure of simian virus 40 at 3.8-Å resolution. Nature 354:278-284. [DOI] [PubMed] [Google Scholar]
- 22.Lin, W., T. Hata, and H. Kasamatsu. 1984. Subcellular distribution of viral structural proteins during simian virus 40 infection. J. Virol. 50:363-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannova, P., D. Liebl, N. Krauzewicz, A. Fejtova, J. Stokrova, Z. Palkova, B. E. Griffin, and J. Forstova. 2002. Analysis of mouse polyomavirus mutants with lesions in the minor capsid proteins. J. Gen. Virol. 83:2309-2319. [DOI] [PubMed] [Google Scholar]
- 24.Montross, L., S. Watkins, R. B. Moreland, H. Mamon, D. L. D. Caspar, and R. L. Garcea. 1991. Nuclear assembly of polyomavirus capsids in insect cells expressing the major capsid protein VP1. J. Virol. 65:4991-4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakanishi, A., D. Shum, H. Morioka, E. Otsuka, and H. Kasamatsu. 2002. Interaction of Vp3 nuclear localization signal with importin α2/β1 heterodimer directs the nuclear entry of infecting SV40. J. Virol. 76:9368-9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okun, M. M., P. M. Day, H. L. Greenstone, F. P. Booy, D. R. Lowy, J. T. Schiller, and R. B. Roden. 2001. L1 interaction domains of papillomavirus L2 necessary for viral genome encapsidation. J. Virol. 75:4332-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahli. R., R. Freund, T. Dubensky, R. Garcea, R. Bronson, and T. Benjamin. 1993. Defect in entry and altered pathogenicity of a polyoma virus mutant blocked in VP2 myristylation. Virology 192:142-153. [DOI] [PubMed] [Google Scholar]
- 28.Stamatos, N. M., S. Chakrabarti, B. Moss, and, J. D. Hare. 1987. Expression of polyomavirus virion proteins by a vaccinia virus vector: association of VP1 and VP2 with the nuclear framework. J. Virol. 61:516-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stauffer, Y., K. Raj, K. Masternak, and P. Beard. 1998. Infectious human papillomavirus type 18 pseudovirions. J. Mol. Biol. 283:529-536. [DOI] [PubMed] [Google Scholar]
- 30.Stehle, T., S. J. Gamblin, Y. Yan, and S. C. Harrison. 1996. The structure of simian virus 40 refined at 3.1 Å resolution. Structure 4:165-182. [DOI] [PubMed] [Google Scholar]
- 31.Stehle, T., Y. Yan, T. L. Benjamin, and S. C. Harrison. 1994. Structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature 369:160-163. [DOI] [PubMed] [Google Scholar]
- 32.Zhao, K. N., X. Y. Sun, I. H. Frazer, and J. Zhou. 1998. DNA packaging by L1 and L2 capsid proteins of bovine papillomavirus type 1. Virology 243:482-491. [DOI] [PubMed] [Google Scholar]