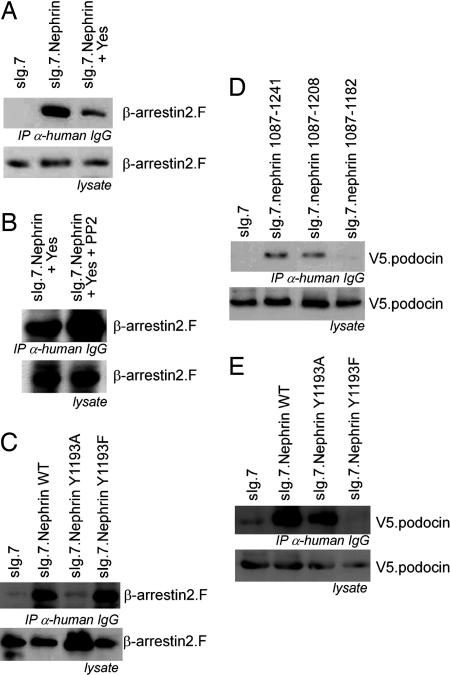
Fig. 3.
Nephrin Y1193 is the switch for β-arrestin2 or podocin interaction with nephrin controlled by phosphorylation. (A) Immunoprecipitation of the nephrin C terminus in the presence of Yes decreases the interaction with β-arrestin2. (B) Addition of PP2 reverses the Yes-mediated attenuation of the interaction between nephrin and β-arrestin2. (C) Point mutation of the Y1193 within the nephrin C terminus modulates the interaction with β-arrestin2. The mutation of tyrosine 1193 to alanine (Y1193A) attenuates the interaction with β-arrestin2. The nephrin mutation of tyrosine 1193 to phenylalanine (Y1193F) interacts with β-arrestin2 as strong as nephrin wild type. (D) The podocin interaction with the nephrin C terminus maps to the same nephrin region that regulates the interaction with β-arrestin2. Immunoprecipitation of the nephrin C terminus and its truncations (as labeled) delineates the podocin interaction motif to the nephrin amino acids 1177–1208, the same region that regulates the interaction with β-arrestin2. (E) In contrast to β-arrestin2, podocin interacts with nephrin Y1193A but not with nephrin Y1193F. (Overexpression experiments were done in HEK293T.)