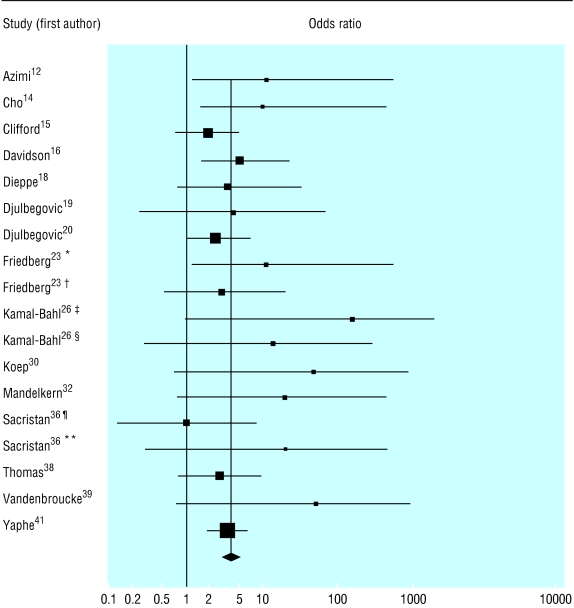
Fig 2.
Source of funding and outcome in pharmacoeconomic analyses, clinical trials, and meta-analyses of clinical trials of drug treatments; for references see bmj.com (*Favourable qualitative results; †Overstatement of quantitative results; ‡Reporting possibility of cost effectiveness or cost savings of prophylaxis in entire high risk infant population either in point estimates or sensitivity analysis; §Reporting cost effectiveness or cost savings in either entire high risk populations or specific infant subgroups compared across studies; ¶Analyses reported in general medical journals; **Analyses reported in Pharmacoeconomics)