Figure 2.
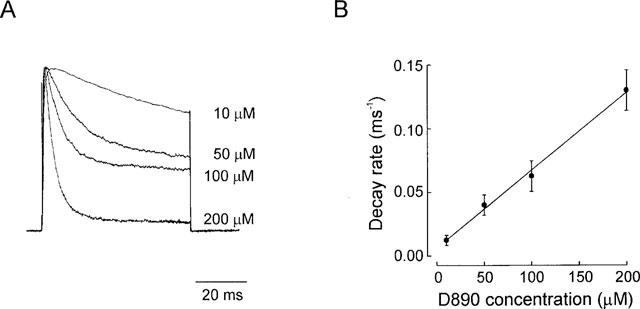
Evaluation of the kinetics of block. (A) Typical IK(DR) (normalized) elicited by depolarizing steps to +120 mV (VH=−70 mV) in presence of varying concentrations of D890 in the pipette. (B) The reciprocal of the time constant of the rate of block, obtained by single exponential fit of the decay phase of the current, is plotted against D890 concentration. Data points (each averaged from 3–5 experiments) were well fitted by a linear regression, indicating that block occurs with molecularity of 1.kon and koff, given by the slope and y-intercept of the interpolated line, were 0.61±0.03 ms−1.mM−1 and 6.4×10−4±0.0017 ms−1, respectively. Bath solution, Na-PSS.
