Abstract
The antidepressant efficacy of selective serotonin reuptake inhibitors (SSRIs) might be enhanced by co-administration of 5-HT1A receptor antagonists. Thus, we have recently shown that the selective 5-HT1A receptor antagonist, WAY 100635, blocks the inhibitory effect of an SSRI on 5-HT cell firing, and enhances its ability to elevate extracellular 5-HT in the forebrain. Here we determined whether the β-adrenoceptor/5-HT1A receptor ligands (±)-pindolol, (−)-tertatolol and (−)-penbutolol, interact with paroxetine in a similar manner.
Both (−)-tertatolol (2.4 mg kg−1 i.v.) and (−)-penbutolol (2.4 mg kg−1 i.v.) enhanced the effect of paroxetine (0.8 mg kg−1 i.v.) on extracellular 5-HT in the frontal cortex, whilst (±)-pindolol (4 mg kg−1 i.v.) did not. (−)-Tertatolol (2.4 mg kg−1 i.v.) alone caused a slight increase in 5-HT however, (−)-penbutolol (2.4 mg kg−1 i.v.) alone had no effect.
In electrophysiological studies (−)-tertatolol (2.4 mg kg−1 i.v.) alone had no effect on 5-HT cell firing but blocked the inhibitory effect of paroxetine. In contrast, (−)-penbutolol (0.1–0.8 mg kg−1 i.v.) itself inhibited 5-HT cell firing, and this effect was reversed by WAY 100635 (0.1 mg kg−1 i.v.). We have recently shown that (±)-pindolol inhibits 5-HT cell firing via a WAY 100635-sensitive mechanism.
Our data suggest that (−)-tertatolol enhances the effect of paroxetine on forebrain 5-HT via blockade of 5-HT1A autoreceptors which mediate paroxetine-induced inhibition of 5-HT cell firing. In comparison, the mechanisms by which (−)-penbutolol enhances the effect of paroxetine on extracellular 5-HT is unclear, since (−)-penbutolol itself appears to have agonist properties at the 5-HT1A autoreceptor. Indeed, the agonist action of (±)-pindolol at 5-HT1A autoreceptors probably explains its inability to enhance the effect of paroxetine on 5-HT in the frontal cortex.
Overall, our data suggest that both (−)-tertatolol and (−)-penbutolol are superior to (±)-pindolol in terms of enhancing the effect of an SSRI on extracellular 5-HT. Both (−)-tertatolol and (−)-penbutolol are worthy of investigation for use as adjuncts to SSRIs in the treatment of major depression.
Keywords: SSRI, antidepressant, paroxetine, β-adrenoceptor/5-HT1A receptor ligands, (±)-pindolol, (−)-tertatolol, (−)-penbutolol, 5-HT cell firing, extracellular 5-HT
Introduction
Increasing 5-HT transmission in the forebrain is proposed to underlie the therapeutic action of many antidepressant drugs including selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), and some tricyclic antidepressants (TCAs) (Delgado et al., 1990; Blier & de Montigny, 1994). However, indirect 5-HT autoreceptor activation by such drugs may actually hamper their therapeutic efficacy. Thus, current strategies aimed at improving antidepressant drug efficacy include the co-administration of 5-HT autoreceptor antagonists (Artigas et al., 1994; Blier & Bergeron, 1995).
The rationale behind this strategy is the finding in animal studies that 5-HT1A autoreceptor antagonists prevent 5-HT reuptake inhibitors (both selective and non-selective), and MAOIs, from indirectly inhibiting 5-HT cell firing, via activation of 5-HT1A autoreceptors (Sheard et al., 1972; Scuvée-Moreau & Dresse, 1979; Chaput et al., 1986; Aghajanian et al., 1970; Gartside et al., 1995; 1997; Sharp et al., 1997a). Hence, the ability of SSRIs and MAOIs to elevate extracellular 5-HT in the forebrain can be enhanced by co-administration of a selective 5-HT1A receptor antagonist such as WAY 100635 (Gartside et al., 1995; Hjorth et al., 1996; Romero et al., 1996a; Sharp et al., 1997a).
Although no selective 5-HT1A receptor antagonist drugs are presently available for use in humans, there are several non-selective β-blockers with high affinity for the 5-HT1A receptor (Middlemiss et al., 1977; see also Sánchez et al., 1996a), and these may be useful alternatives. Indeed, (±)-pindolol has already been used in combination with SSRIs, in a number of clinical studies. However, whilst early open trials found a markedly improved antidepressant effect of combined (±)-pindolol/SSRI treatment (Artigas et al., 1994; Blier & Bergeron, 1995), more recent double-blind placebo-controlled trials have produced mixed results (Berman et al., 1997; Moreno et al., 1997; Perez et al., 1997; Zanardi et al., 1997; for review see McAskill et al., 1998).
Against this background, it is of note that emerging evidence indicates that (±)-pindolol has partial agonist properties at the 5-HT1A autoreceptor (Hjorth & Carlsson, 1986; Clifford et al., 1998; Newman-Tancredi et al., 1998). This feature may mean that (±)-pindolol would be of limited value as an adjunct to antidepressant therapy.
In the present study, we have selected two further β-adrenoceptor antagonists, (−)-tertatolol, and (−)-penbutolol, with reported antagonist properties at the 5-HT1A receptor (Hjorth & Sharp, 1993; Jolas et al., 1993; Prisco et al., 1993; Sánchez et al., 1996a,1996b), and compared them with (±)-pindolol in two respects; firstly, their ability to enhance the effect of an SSRI on extracellular 5-HT in the forebrain and secondly, their ability to block the inhibitory effect of an SSRI on 5-HT cell firing in the dorsal raphé nucleus (DRN).
Some of these data have been presented in preliminary form to the British Pharmacological Society (Gartside et al., 1998).
Methods
Animals
Male Sprague-Dawley rats (265–305 g) (Harlan-Olac, Bicester, U.K.) were housed in groups of up to six under controlled conditions of temperature (21°C) and humidity (50%), in a 12 h light/dark cycle (lights on 08.00 h). Animals were allowed food and water ad libitum.
General stereotaxic surgical procedures
Rats were anaesthetized with chloral hydrate (500 mg kg−1 i.p. plus supplementary doses given as necessary), and were placed in a stereotaxic frame (David Kopf) with the incisor bar set at −3.3 mm. The skull was exposed, and a burr hole drilled for implantation of a microdialysis probe or recording electrode. The body temperature of the animals was maintained at 35.0±0.5°C by means of a homeothermic blanket with rectal probe. A lateral tail vein was cannulated, using a 25 gauge hypodermic needle, for i.v. administration of drugs.
Microdialysis experiments
Microdialysis probes of a concentric design, with a 4 mm window of Hospal membrane, were constructed in house (Sharp & Zetterström, 1992). Probes were implanted into the frontal cortex (AP +3.0 mm; ML 3.2 mm; DV 5.0 mm from bregma and dura (Paxinos & Watson, 1986), and were perfused (at 2 μl min−1) with an artificial CSF (composition (mM): NaCl 140, KCl 3, CaCl2 2.4, MgCl2 1.0, Na2HPO4 1.2, NaH2PO4 0.27, glucose 7.2, pH 7.4). Dialysate samples were collected every 20 min and assayed for 5-HT by h.p.l.c. with electrochemical detection (ED). Once baseline levels of 5-HT had stabilized, drugs were given (i.v.) as single bolus doses in a volume of 1 ml kg−1. (±)-Pindolol, (−)-penbutolol, (−)-tertatolol, or vehicle was given 10 min before paroxetine (or vehicle), and 5-HT was measured for a further 2 h.
H.p.l.c.-ED
Immediately following collection, dialysates were analysed for 5-HT by h.p.l.c.-ED. Samples were separated on a Microsorb™ column (C18, Rainin) with a mobile phase (methanol 12.5%, disodium EDTA 0.03%, NaH2PO4 127 mM, octane sulphonic acid 15 μM; pH 4.0) pumped at 1.2 ml min−1. Detection of 5-HT was by a potentiometer (BAS LC-4B/C) with the working electrode (glassy carbon) set at +0.7 V.
Electrophysiological recordings
Single-barrelled glass microelectrodes were made from glass capillary tube (external Ø 2.5 mm) (Clarke Electromedical, Pangbourne, U.K.). Electrodes were broken back to obtain an external tip diameter of ≈2 μm, and were then filled with a solution of 2 M NaCl containing 2% Pontamine Sky Blue. The in vitro impedance of electrodes was 4–8 MΩ. Electrodes were initially implanted to a depth of 4.5 mm below dura, and then lowered into the DRN (AP −7.8 mm; ML 0 mm, from bregma (Paxinos & Watson, 1986)) by means of an hydraulic microdriver (David Kopf).
The signal was amplified (×1000) and filtered (300–3000 Hz band pass), and was fed to an oscilloscope, a chart recorder, an audio speaker, and a digital audio tape recorder. Presumed 5-HT neurones in the DRN, having characteristics similar to the immunohistologically identified 5-HT-containing neurones of the DRN described by Aghajanian & VanderMaelen (1982), were encountered between 5.0 and 6.5 mm below the dura surface. The neurones were spontaneously active, and fired solitary (positive/negative) action potentials of long duration, in a slow and regular pattern. Extracellular recordings were made from one cell per animal.
The baseline firing activity of each neurone was recorded for at least 3 min after which time, drugs were given (i.v.). At the end of each electrophysiological experiment the brain was removed, post-fixed in 4% paraformaldehyde, and subsequently sectioned using a vibratome. Sections were stained with cresyl violet and the position of the electrode tip (marked by ejection of Pontamine Sky Blue) was determined by microscopic inspection. All of the recorded neurones included in the present study were found to be within the DRN.
Drugs
The following drugs (sources in brackets) were used: chloral hydrate (Sigma), (−)-penbutolol HCl (Roussel, France), (±)-pindolol HCl (Sigma), (−)-tertatolol HCl (Servier, Neuilly-sur-Marne, France), paroxetine HCl (SmithKline Beecham, Harlow, U.K.), WAY 100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl) cyclohexane carboxamide 3HCl (Wyeth Research, Maidenhead, U.K.). (±)-Pindolol was dissolved in a drop of glacial acetic acid, made up to volume with 5% glucose, and the pH adjusted to 4.5 with 5 M NaOH, all other drugs were dissolved in distilled water.
Data presentation and statistics
For microdialysis experiments, dialysate levels of 5-HT are expressed as a percentage of the absolute amount of 5-HT in the sample collected immediately prior to administration of paroxetine or its vehicle i.e. defined as t=0 in the figures. The absolute amount of 5-HT at t=0 is given in the figure legends as fmol sample−1. Drug effects were assessed using 2-way ANOVA with repeated measures.
In the electrophysiological studies, basal firing rate refers to the firing rate in the 60 s immediately before drug administration, and is presented as spikes 10 s−1. 5-HT cell firing after drug administration refers to the firing rate in a 30 s period (starting 90 s after administration of a drug) expressed as a percentage of the basal firing rate. Data are presented as mean±s.e.mean (n) values. Significance at the 95% level is reported.
Results
Effects of (±)-pindolol, (−)-tertatolol, and (−)-penbutolol on 5-HT in dialysates of the frontal cortex: in combinatiaon with paroxetine and alone
Paroxetine alone (0.8 mg kg−1) did not increase levels of 5-HT in dialysates of the frontal cortex during the 2 h duration of the experiment. This was apparent in three separate vehicle pretreated groups of animals (see Figures 1, 2 and 3). Pretreatment with 4 mg kg−1 (±)-pindolol did not alter the effect of paroxetine (0.8 mg kg−1) on dialysate 5-HT over the duration of the experiment (Figure 1). Thus, statistical analysis of 5-HT levels in the (±)-pindolol/paroxetine and vehicle/paroxetine groups showed no significant differences.
Figure 1.
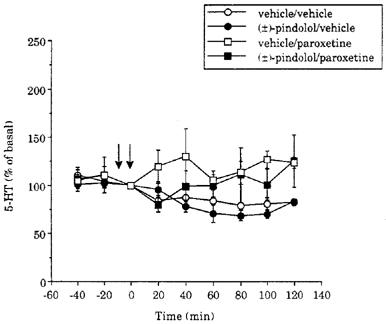
Time course of the effect of paroxetine (0.8 mg kg−1 i.v.) on 5-HT levels in dialysates of the frontal cortex in vehicle- and (±)-pindolol (4 mg kg−1) pretreated animals. The vehicle was acetic acid/glucose. Drugs were given as indicated by the arrows. Basal levels of 5-HT were 11±2 (5), 25±5 (4), 15±2 (6) and 23±5 (6) fmol sample−1, in the groups of animals which received vehicle/vehicle, (±)-pindolol/vehicle, vehicle/paroxetine and (±)-pindolol/paroxetine, respectively.
Figure 2.
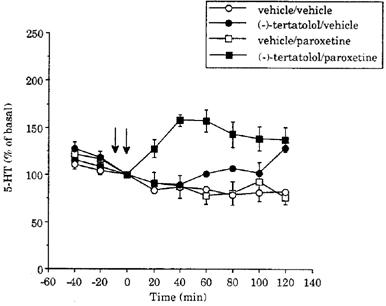
Time course of the effect of paroxetine (0.8 mg kg−1 i.v.) on 5-HT levels in dialysates of the frontal cortex in vehicle- and (−)-tertatolol- (2.4 mg kg−1) pretreated animals. The vehicle was distilled water. Drugs were given as indicated by the arrows. Basal levels of 5-HT were 11±2 (5), 17±3 (4), 17±3 (5) and 19±3 (5), and fmol sample−1, in the groups of animals which received vehicle/vehicle, (−)-tertatolol/vehicle, vehicle/paroxetine and (−)-tertatolol/paroxetine, respectively.
Figure 3.
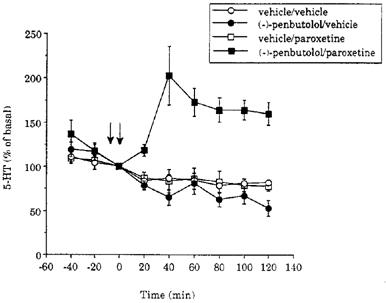
Time course of the effect of paroxetine (0.8 mg kg−1 i.v.) on 5-HT levels in dialysates of the frontal cortex in vehicle- and (−)- penbutolol- (2.4 mg kg−1) pretreated animals. The vehicle was distilled water. Drugs were given as indicated by the arrows. Basal levels of 5-HT were 11±2 (5), 10±1 (5), 14±2 (6) and 9±1 (6) fmol sample−1, in the groups of animals which received vehicle/vehicle, (−)-penbutolol/vehicle, vehicle/paroxetine and (−)-penbutolol/paroxetine, respectively. Data for the vehicle/vehicle treated group are taken from Figure 2.
In additional experiments it was found that dialysate 5-HT did not increase when the order of administration of pindolol/paroxetine was reversed (i.e. 4 mg kg−1 (±)-pindolol 60 min after 0.8 mg kg−1 paroxetine, n=3 rats). Since 4 mg kg−1 (±)-pindolol may be supramaximal in terms of its inhibitory effect on 5-HT cell-firing (Clifford et al., 1998), we pretreated with a lower dose of (±)-pinodolol (0.8 mg kg−1) but in these animals 0.8 mg kg−1 paroxetine also failed to increase 5-HT (n=4 rats).
The lack of effect of (±)-pindolol was in contrast to the marked increase in 5-HT in response to paroxetine (0.8 mg kg−1) in rats pretreated with (−)-tertatolol (2.4 mg kg−1). Thus, in the (−)-tertatolol pretreated group, 5-HT levels rose to approximately 160% of basal and remained elevated over the 2 h duration of the experiment (Figure 2). Statistical analysis of the two groups revealed a significant main effect of treatment (F(1,8)=16.4; P=0.0037), as well as a significant treatment ×time interaction (F(6,48)=6.3; P=0.0001).
(−)-Tertatolol (2.4 mg kg−1) alone induced a small but significant increase in dialysate 5-HT when compared to vehicle (Figure 2). The statistical analysis revealed a main effect of treatment (F(1,7)=10.2; P=0.0153), as well as a significant treatment×time interaction (F(6,42)=3.0; P=0.0145) between the (−)-tertatolol/vehicle and vehicle/vehicle groups.
As shown in Figure 3, the effect of paroxetine (0.8 mg kg−1) on dialysate levels of 5-HT was also enhanced by pretreatment with (−)-penbutolol (2.4 mg kg−1); dialysate 5-HT increased to more than 200% of basal, and levels remained elevated for at least 2 h. Statistical analysis of the vehicle/paroxetine and (−)-penbutolol/paroxetine treated groups revealed significant main effects of time (F(6,54)=3.7; P=0.0039) and treatment (F(1,9)=35.1; P=0.0002), as well as a significant interaction (F(6,54)=5.3; P=0.0002). When given alone, (−)-penbutolol (2.4 mg kg−1) did not alter dialysate 5-HT levels compared to vehicle (Figure 3).
Effect of (−)-tertatolol and (−)-penbutolol on 5-HT cell firing in the DRN
(−)-Tertatolol (0.8–2.4 mg kg−1) did not alter the firing rate of 5-HT neurones in the DRN (Figures 4 and 5a). In contrast, (−)-penbutolol (0.1–0.8 mg kg−1) caused marked inhibition of the firing of 5-HT neurones in the DRN (n=8) (Figure 4). This effect was dose-dependent, although there was some variation in the magnitude of effect. Thus, in one cell 0.2 mg kg−1 (−)-penbutolol caused a near complete inhibition of firing (see Figure 5b). At the other extreme, one cell showed only 12% inhibition after 0.8 mg kg−1 (−)-penbutolol (and 30% inhibition after 2.4 mg kg−1) (see Figure 5c). The inhibitory effect of (−)-penbutolol was reversed by WAY 100635 (0.1 mg kg−1) in four of five cases in which it was tested (see Figure 5b and d for examples), although in one case this effect was short-lived.
Figure 4.
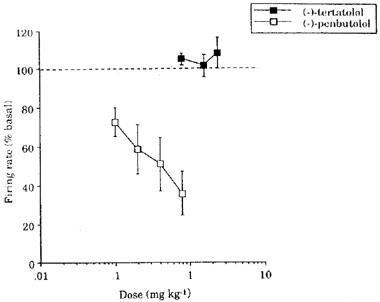
Effect of increasing doses of (−)-tertatolol and (−)-penbutolol on the firing rate of 5-HT cells in the DRN. Basal firing rates were 16.3±2.5 (9) and 8.7±2.0 (9) spikes 10 s−1 in the groups treated with (−)-tertatolol and (−)-penbutolol respectively.
Figure 5.
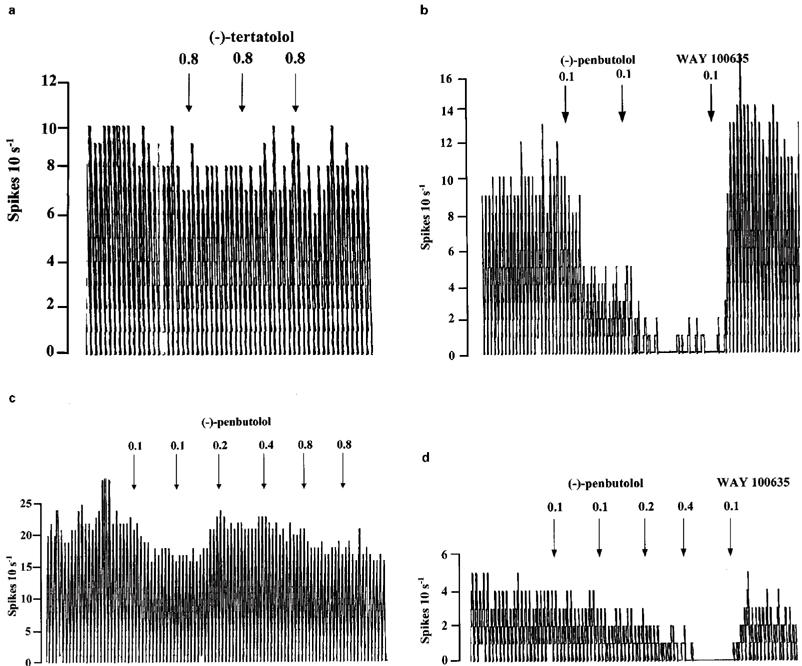
Rate meter recordings showing the effects of (−)-tertatolol and (−)-penbutolol on the firing rate of individual 5-HT cells in the DRN. Drugs were given as indicated by the arrows. (a) Shows a cell in which (−)-tertatolol did not influence the firing rate. (b), (c) and (d) show cells which were inhibited by (−)-penbutolol. Note that the cell shown in (c) is much less sensitive to inhibition by (−)-penbutolol than those in (b) and (d). Note also the reversal of the inhibitory effect of (−)-penbutolol by WAY 100635 shown in (b) and (d).
Effect of (−)-tertatolol on 5-HT cell firing in the DRN: interaction with paroxetine
Paroxetine (0.1–0.8 mg kg−1) inhibited 5-HT cells in the DRN in a dose-dependent manner with a dose of 0.8 mg kg−1 causing near complete inhibition of firing (Figures 6 and 7a). In the group of animals pretreated with (−)-tertatolol (total dose 2.4 mg kg−1), the paroxetine dose-response curve was shifted to the right such that 0.8 mg kg−1 paroxetine caused only approximately 40% inhibition of cell firing in these animals (Figures 6 and 7b).
Figure 6.
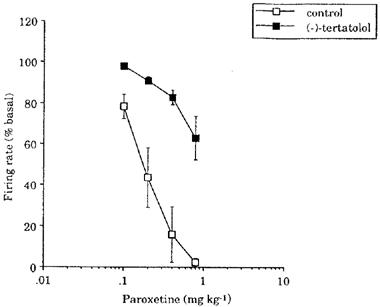
Effect of increasing doses of paroxetine on the firing rate of 5-HT cells in the DRN in naïve animals (control) and animals pretreated with (−)-tertatolol (2.4 mg kg−1). Basal firing rates were 7.7±0.9 (6) 19.7±4.7 (4) spikes 10 s−1 in the control and (−)-tertatolol pretreated groups respectively.
Figure 7.
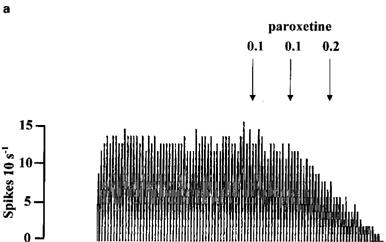
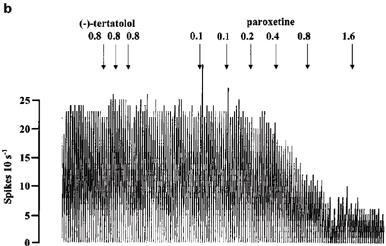
Rate meter recordings showing the effect of paroxetine on the firing rate of individual 5-HT cells in the DRN. Drugs were given as indicated by the arrows. (a) Shows the effect of paroxetine in a control cell. (b) Shows the effect of paroxetine in a (−)-tertatolol-pretreated cell.
In the animals which received paroxetine first (n=8) total dose (0.2–0.8 mg kg−1), attempts were made to reverse the effect of paroxetine by subsequent administration of (−)-tertatolol and/or WAY 100635. In four cells, (−)-tertatolol partially reversed the effect of paroxetine, although the magnitude of effect was variable. Thus, in one cell (−)-tertatolol (2.4 mg kg−1) caused near full reversal of the inhibition (Figure 8a). In two cells (−)-tertatolol caused a return to approximately 50% of the basal firing (in one of these cells, subsequent administration of WAY 100635 returned the firing rate to approximately 100% (see Figure 8b), in the other WAY 100635 was without further effect. In one other cell, a brief burst of spikes was seen after (−)-tertatolol. (−)-Tertatolol (2.4 mg kg−1) (see figure 8c) failed to reverse the inhibitory effect of paroxetine in four further cells. In two of these cells some reversal was induced by subsequent administration of WAY 100635 (0.1 mg kg−1) whilst, in the remaining two cells, firing activity could not be restored by WAY 100635 (three doses of 0.1 mg kg−1).
Figure 8.
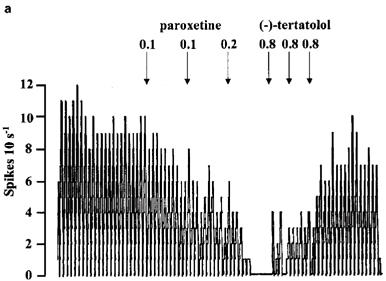
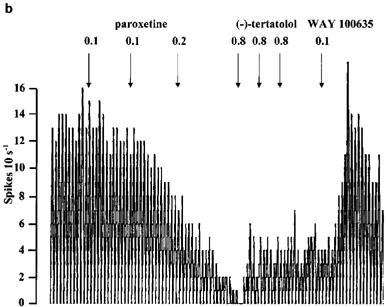
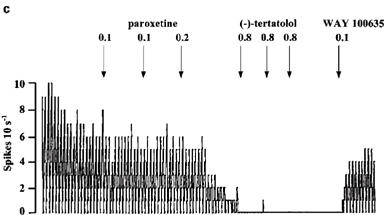
Rate meter recordings showing the effect of (−)-tertatolol and WAY 100635 on the inhibitory response to paroxetine in individual 5-HT cells in the DRN. Drugs were given as indicated by the arrows. (a) Shows a cell in which (−)-tertatolol caused near complete reversal of the effect of paroxetine. (b) Shows a cell in which the reversal by (−)-tertatolol was partial whilst (c) shows a cell in which (−)-tertatolol was ineffective. Note that subsequent administration of WAY 100635 reversed the inhibitory effect of paroxetine in the cells shown in (b) and (c).
Due to the inhibition of 5-HT cell firing induced by (−)-penbutolol, and (±)-pindolol alone, attempts were not made to reverse the inhibitory effect of paroxetine with these drugs.
Discussion
The adjunctive use of 5-HT1A receptor antagonists may be a means by which to improve the antidepressant efficacy of SSRIs and other antidepressants (Artigas et al., 1996). We have recently shown that the silent and selective 5-HT1A receptor antagonist, WAY 100635 (Forster et al., 1995), blocks the inhibitory effect of paroxetine on 5-HT cell firing, and enhances its 5-HT elevating effect in the frontal cortex (Gartside et al., 1995). Here we have examined whether the 5-HT1A/β-adrenoceptor ligands, (±)-pindolol, (−)-tertatolol, and (−)-penbutolol, share the properties of WAY 100635 when combined with paroxetine.
In our microdialysis studies we found that both (−)-tertatolol, and (−)-penbutolol enhanced the effect of paroxetine on extracellular 5-HT in the frontal cortex, whereas (±)-pindolol was without significant effect. Therefore, whilst (−)-tertatolol, and (≈)-penbutolol mimicked WAY 100635 in terms of enhancing the effect of paroxetine on extracellular 5-HT, (±)-pindolol clearly did not. However, further experiments on the effect of (−)-tertatolol, and (−)-penbutolol on the firing rate of 5-HT neurones, and specifically their ability to block the inhibitory effect of paroxetine, revealed a more complex picture (see below).
The finding of the present study that (±)-pindolol failed to enhance the effect of paroxetine on extracellular 5-HT in the frontal cortex is consistent with our recent report that this drug has agonist activity at somatodendritic 5-HT1A autoreceptors (Clifford et al., 1998). Thus, it would seem logical that an inhibitory action of (±)-pindolol on 5-HT cell firing and release would oppose any paroxetine-induced increase in extracellular 5-HT in the forebrain. Although some studies have shown that pindolol enhances the effect of SSRIs (including paroxetine) on extracellular 5-HT (Hjorth, 1996; Romero et al., 1996b; Maione et al., 1997), our conclusion is that, compared to WAY 100635, (−)-tertatolol, and (−)-penbutolol, (±)-pindolol is, at best, weakly active in its interaction with paroxetine.
The explanation for our failure to confirm reports that pindolol enhances the effect of SSRIs on extracellular 5-HT is not immediately clear. Although some studies find that (±)-pindolol causes a rise in 5-HT when administered after the SSRI (Hjorth, 1996), in our hands order of administration of (±)-pindolol/paroxetine did not change the outcome (see Results). Moreover, our recent studies have ruled out the likelihood that it is due to factors such as dose or enantiomer of pindolol used, brain region studied (hippocampus versus frontal cortex), or presence of anaesthesia (see Hughes & Sharp, 1998).
(−)-Tertatolol, in contrast to (±)-pindolol, enhanced the ability of paroxetine to increase cortical extracellular 5-HT. Moreover, our present electrophysiological studies showed that (−)-tertatolol antagonizes the inhibitory effect of paroxetine on 5-HT cell firing. Thus, we found that (−)-tertatolol shifted the paroxetine dose-response curve to the right, and also reversed the effect of paroxetine in some cases. Taken together, these data are consistent with the blockade of somatodendritic 5-HT1A receptors by (−)-tertatolol.
Several studies have shown that (−)-tertatolol antagonizes the effects of the 5-HT1A receptor agonists in animal models, including inhibition of DRN 5-HT cell firing, as well as secretion of corticosterone, hypothermia, and flat body posture (Jolas et al., 1993; Lejeune et al., 1993; Prisco et al., 1993; Gobert et al., 1995). Some studies suggest that (−)-tertatolol may have 5-HT1A receptor agonist-like activity in vivo (Millan et al., 1993; Andrews et al., 1994; Sánchez et al., 1996a). However, the finding reported here, and by others (Jolas et al., 1993; Lejeune et al., 1993), that the drug does not inhibit 5-HT cell firing would argue against this since this measure is well recognized to be sensitive to even low efficacy 5-HT1A receptor agonists.
Although (−)-tertatolol also has moderate affinity for the rat 5-HT1B receptor, at which it is thought to act as an antagonist (Langlois et al., 1993; Prisco et al., 1993; Arvieu et al., 1996; Assié & Koek, 1996), it is unlikely that 5-HT1B receptor antagonism alone can explain the enhancement of extracellular 5-HT seen here. Thus, we have previously found that 5-HT1B receptor antagonists only elevate extracellular 5-HT when levels of 5-HT in the synapse are already raised (Sharp et al., 1997b; and unpublished data), conditions which do not occur following the dose of paroxetine given here. Nevertheless, in the rat, 5-HT1B receptor antagonism may contribute to the effect of (−)-tertatolol if, as suggested by our data, it effectively blocks 5-HT1A receptors as well.
(−)-Penbutolol, like (−)-tertatolol, enhanced the ability of paroxetine to increase extracellular 5-HT in the frontal cortex. Several previous microdialysis studies have also reported that (−)-penbutolol enhances the effects of SSRIs on extracellular 5-HT, an effect which has been consistently attributed to 5-HT1A receptor antagonism (Hjorth, 1993; Gundlah et al., 1997; Invernizzi et al., 1997). Interestingly however, in our electrophysiological experiments, we found that (−)-penbutolol appeared to act as an agonist at the somatodendritic 5-HT1A autoreceptor. Thus, we found that (−)-penbutolol itself decreased 5-HT cell firing, in a WAY 100635-sensitive manner. An earlier preliminary report also described the inhibition of 5-HT cell firing following (−)-penbutolol (VanderMaelen & Braselton, 1992). It is likely that this effect of (−)-penbutolol is due to a direct agonist action at 5-HT1A autoreceptors since we find that the drug does not increase extracellular 5-HT in the DRN at doses which inhibit 5-HT cell firing (Gartside, unpublished observations).
The action of (−)-penbutolol to inhibit 5-HT cell firing is difficult to reconcile with the enhancement of the effect of paroxetine observed in the microdialysis experiment. Like (−)-tertatolol, (−)-penbutolol is an antagonist at rat 5-HT1B receptors (Schlicker et al., 1992; Hjorth & Sharp, 1993; Sánchez et al., 1996b) however, as discussed above, this action is unlikely to explain the drug's ability to enhance the effect of paroxetine on extracellular 5-HT. It is not likely that the 5-HT cells inhibited by (−)-penbutolol are not those which project to the frontal cortex since there is both anatomical and functional evidence that the DRN is the source of afferentation to the frontal cortex (Kosofsky & Molliver, 1987; McQuade & Sharp, 1997).
One final possibility that deserves mention is that the inhibitory effect of (−)-penbutolol on 5-HT cell firing might be short-lived, and that during the time scale of the dialysis study, 5-HT1A receptor antagonist properties of (−)-penbutolol become apparent. Interestingly, in the preliminary report by VanderMaelen & Braselton (1992) it was found that a 10 min pretreatment with (−)-penbutolol did not prevent 8-OH-DPAT-induced inhibition of 5-HT cell firing whereas a 60 min pretreatment did. Blockade of the 5-HT1A autoreceptor may therefore play a role in the increase in extracellular 5-HT detected following the (−)-penbutolol/paroxetine combination.
Interestingly, (−)-penbutolol inhibited all 5-HT cells on which it was tested although there was some variability in the magnitude of effect (c.f. VanderMaelen & Braselton, 1992). In our previous study we found that (±)-pindolol inhibited only a subpopulation (approximately 50%) of 5-HT cells, those with lower basal firing rates being (±)-pindolol-sensitive (Clifford et al., 1998). The present study, failed to reveal a ‘(−)-penbutolol-insensitive' population of cells, although the one cell which was notably less sensitive to (−)-penbutolol (that shown in Figure 5c) had the highest firing rate of the cells tested. It may be that a study of a larger number of cells with a broader range of firing rates might reveal a ‘(−)-penbutolol-insensitive' population.
In summary, our in vivo microdialysis studies revealed that (−)-tertatolol and (−)-penbutolol enhanced the effect of paroxetine on extracellular 5-HT in the cortex, whilst (±)-pindolol did not. Examination of the mechanisms underlying the effects of (−)-tertatolol and (−)-penbutolol suggested that the effect of (−)-tertatolol was mediated by 5-HT1A receptor blockade, whilst the mechanism underlying the action of (−)-penbutolol is somewhat unclear but might also involve a similar mechanism. Our data suggest that both (−)-tertatolol and (−)-penbutolol are superior to (±)-pindolol in terms of enhancing the effect of an SSRI on extracellular 5-HT. Therefore, both drugs are worthy of investigation as adjuncts to SSRIs in the therapy of major depression.
Acknowledgments
This work was supported by grants from the M.R.C. and the University of Oxford. We are grateful to Roussel, Servier, SmithKline Beecham, and Wyeth for generous gifts of drugs.
Abbreviations
- 5-HT
5-hydroxytryptamine
- SSRI
selective serotonin reuptake inhibitor
- MAOI
monoamine oxidase inhibitor
- TCA
tricyclic antidepressant
- DRN
dorsal raphé nucleus
- CSF
cerebrospinal fluid
- h.p.l.c.-ED
high performance liquid chromatography with electrochemical detection
- EDTA
ethylenediaminetetra acetic acid
- AP
anterior-posterior
- ML
medial-lateral
References
- AGHAJANIAN G.K., GRAHAM A.W., SHEARD M.H. Serotonin-containing neurons in brain: depression of firing by monoamine oxidase inhibitors. Science. 1970;169:1100–1102. doi: 10.1126/science.169.3950.1100. [DOI] [PubMed] [Google Scholar]
- AGHAJANIAN G.K., VANDERMAELEN C.P. Intracellular identification of central noradrenergic and serotonergic neurons by a new double labeling procedure. J. Neurosci. 1982;2:1786–1792. doi: 10.1523/JNEUROSCI.02-12-01786.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDREWS N., HOGG S., GONZALEZ L.E., FILE S.E. 5-HT1A receptors in the median raphé nucleus and dorsal hippocampus may mediate anxiolytic and anxiogenic behaviours respectively. Eur. J. Pharmacol. 1994;264:259–264. doi: 10.1016/0014-2999(94)00473-0. [DOI] [PubMed] [Google Scholar]
- ARTIGAS F., PEREZ V., ALVAREZ E. Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch. Gen. Psychiatry. 1994;51:248–251. doi: 10.1001/archpsyc.1994.03950030084009. [DOI] [PubMed] [Google Scholar]
- ARTIGAS F., ROMERO L., DE MONTIGNY C., BLIER P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- ARVIEU L., MAUBORGNE A., BOURGOIN S., OLIVER C., FELTZ P., HAMON M., CESSELIN F. Sumatriptan inhibits the release of CGRP and substance P from the rat spinal cord. Neuroreport. 1996;7:1973–1976. doi: 10.1097/00001756-199608120-00023. [DOI] [PubMed] [Google Scholar]
- ASSIÉ M.-B., KOEK W. (−)-Pindolol and (±)-tertatolol affect hippocampal 5-HT levels through mechanisms involving not only 5-HT1A, but also 5-HT1B receptors. Neuropharmacol. 1996;35:213–222. doi: 10.1016/0028-3908(95)00169-7. [DOI] [PubMed] [Google Scholar]
- BERMAN R.M., DARNELL A.M., MILLER H.L., ANAND A., CHARNEY D.S. Effect of pindolol in hastening response to fluoxetine in the treatment of major depression: a double blind, placebo controlled trial. Am. J. Psychiatry. 1997;154:37–43. doi: 10.1176/ajp.154.1.37. [DOI] [PubMed] [Google Scholar]
- BLIER P., BERGERON R. Effectiveness of pindolol with selected antidepressant drugs in the treatment of major depression. J. Clin. Psychopharmacol. 1995;15:217–222. doi: 10.1097/00004714-199506000-00011. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C. Current advances and trends in the treatment of depression. Trends Pharmacol. Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- CHAPUT Y., DE MONTIGNY C., BLIER P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn-Schmiedeberg's Arch. Pharmacol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- CLIFFORD E.M., GARTSIDE S.E., UMBERS V., COWEN P.J., HAJÓS M., SHARP T. Electrophysiological and neurochemical evidence that pindolol has agonist properties at the 5-HT1A autoreceptor in vivo. Br. J. Pharmacol. 1998;124:206–212. doi: 10.1038/sj.bjp.0701796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELGADO P.L., CHARNEY D.S., PRICE L.H., AGHAJANIAN G.K., LANDIS H., HENINGER G.R. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant induced remission by rapid depletion of plasma tryptophan. Arch. Gen. Psychiatry. 1990;47:411–418. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- FORSTER E.A., CLIFFE I.A., BILL D.J., DOVER G.M., JONES D., REILLY Y., FLETCHER A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY100635. Eur. J. Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- GARTSIDE S.E., UMBERS V., CLIFFORD E.M., SHARP T. Effect of paroxetine in combination with the β-blockers/5-HT1A antagonists (±)-pindolol, (−)-tertatolol and (−)-penbutolol, on extracellular 5-HT in the rat cortex. Br. J. Pharmacol. 1998;123:232P. [Google Scholar]
- GARTSIDE S.E., UMBERS V., HAJÓS M., SHARP T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br. J. Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARTSIDE S.E., UMBERS V., SHARP T. Inhibition of 5-HT cell firing in the DRN by non-selective 5-HT reuptake inhibitors: studies on the role of 5-HT1A autoreceptors and noradrenergic mechanisms. Psychopharmacology. 1997;130:261–268. doi: 10.1007/s002130050238. [DOI] [PubMed] [Google Scholar]
- GOBERT A., LEJEUNE F., RIVET J.-M., AUDINOT V., NEWMAN-TANCREDI A., MILLAN M.J. Modulation of the activity of central serotoninergic neurons by novel serotonin1A receptor antagonists and antagonists: a comparison to adrenergic and dopaminergic neurons in rats. J. Pharmacol. Exp. Ther. 1995;273:1032–1046. [PubMed] [Google Scholar]
- GUNDLAH C., HJORTH S., AUERBACH S.B. Autoreceptor antagonists enhance the effect of the reuptake inhibitor citalopram on extracellular 5-HT: this effect persists after repeated citalopram treatment. Neuropharmacology. 1997;36:475–482. doi: 10.1016/s0028-3908(97)00052-x. [DOI] [PubMed] [Google Scholar]
- HJORTH S. Serotonin 5-HT1A autoreceptor blockade potentiates the ability of the 5-HT reuptake inhibitor citalopram to increase nerve terminal output of 5-HT in vivo: a microdialysis study. J. Neurochem. 1993;60:776–779. doi: 10.1111/j.1471-4159.1993.tb03217.x. [DOI] [PubMed] [Google Scholar]
- HJORTH S. (−)-Pindolol, but not buspirone, potentiates the citalopram-induced rise in extracellular 5-hydroxytryptamine. Eur. J. Pharmacol. 1996;303:183–186. doi: 10.1016/0014-2999(96)00185-9. [DOI] [PubMed] [Google Scholar]
- HJORTH S., BENGTSSON H.J., MILANO S. Raphé 5-HT1A autoreceptors, but not postsynaptic 5-HT1A receptors or β-adrenoceptors, restrain the citalopram-induced increase in extracellular 5-hydroxytryptamine in vivo. Eur. J. Pharmacol. 1996;316:43–47. doi: 10.1016/s0014-2999(96)00779-0. [DOI] [PubMed] [Google Scholar]
- HJORTH S., CARLSSON A. Is pindolol a mixed agonist, antagonist at central serotonin (5-HT) receptors. Eur. J. Pharmacol. 1986;129:131–138. doi: 10.1016/0014-2999(86)90344-4. [DOI] [PubMed] [Google Scholar]
- HJORTH S., SHARP T. In vivo microdialysis evidence for central serotonin1A and serotonin1B autoreceptor blocking properties of the beta-adrenoceptor antagonist (−)-penbutolol. J. Pharmacol. Exp. Ther. 1993;265:707–712. [PubMed] [Google Scholar]
- HUGHES Z.A., SHARP T. Evidence that pindolol lacks the ability to enhance the effect of SSRIs on presynaptic 5-HT function. Br. J. Pharmacol. 1998;125:6P. [Google Scholar]
- INVERNIZZI R., VELASCO C., BRAMANTE M., LONGO A., SAMANIN R. Effect of 5-HT1A receptor antagonists on citalopram-induced increase in extracellular serotonin in the frontal cortex, striatum and dorsal hippocampus. Neuropharmacology. 1997;36:467–473. doi: 10.1016/s0028-3908(97)00060-9. [DOI] [PubMed] [Google Scholar]
- JOLAS T., HAJ-DAHMANE S., LANFUMEY L., FATTACCINI C.M., KIDD E.J., ADRIEN J., GOZLAN H., GUARDIOLA-LEMAITRE B., HAMON M. (−)-Tertatolol is a potent antagonist at pre- and postsynaptic serotonin 5-HT1A receptors in the rat brain. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;347:453–463. doi: 10.1007/BF00166735. [DOI] [PubMed] [Google Scholar]
- KOSOFSKY B.E., MOLLIVER M.E. The serotoninergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal and median raphé nuclei. Synapse. 1987;1:153–168. doi: 10.1002/syn.890010204. [DOI] [PubMed] [Google Scholar]
- LANGLOIS M., BRÉMONT B., ROUSSELLE D., GAUDY F. Structural analysis by the comparative molecular field analysis method of the affinity of β-adrenoceptor blocking agents for 5-HT1A and 5-HT1B receptors. Eur. J. Pharmacol. 1993;244:77–87. doi: 10.1016/0922-4106(93)90061-d. [DOI] [PubMed] [Google Scholar]
- LEJEUNE F., RIVET J.-M., GOBERT A., CANTON H., MILLAN M.J. WAY 100,135 and (−)-tertatolol act as antagonists at both 5-HT1A autoreceptors and postsynaptic 5-HT1A receptors in vivo. Eur. J. Pharmacol. 1993;240:307–310. doi: 10.1016/0014-2999(93)90915-5. [DOI] [PubMed] [Google Scholar]
- MAIONE S., PALAZZO E., PALLOTTA M., LEYVA J., BERRINO L., ROSSI F. Effects of imipramine on raphé nuclei and prefrontal cortex extracellular serotonin levels in the rat. Psychopharmacol. 1997;134:401–405. doi: 10.1007/s002130050477. [DOI] [PubMed] [Google Scholar]
- MCASKILL R., MIR S., TAYLOR D. Pindolol augmentation of antidepressant therapy. Brit. J. Psychiatry. 1998;173:203–208. doi: 10.1192/bjp.173.3.203. [DOI] [PubMed] [Google Scholar]
- MCQUADE R., SHARP T. Functional mapping of dorsal and median raphé 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. J. Neurochem. 1997;69:791–796. doi: 10.1046/j.1471-4159.1997.69020791.x. [DOI] [PubMed] [Google Scholar]
- MIDDLEMISS D.N., BLAKEBOROUGH L., LEATHER S.R. Direct evidence for an interaction of β-adrenergic blockers with the 5-HT receptor. Nature. 1977;267:289–290. doi: 10.1038/267289a0. [DOI] [PubMed] [Google Scholar]
- MILLAN M.J., RIVET J.-M., CANTON H., LE MOAROUILLE-GIRARDON S., GOBERT A. Induction of hypothermia as a model of 5-hydroxytryptamine1A receptor-mediated activity in the rat: a pharmacological characterization of the actions of novel agonists and antagonists. J. Pharmacol. Exp. Ther. 1993;264:1364–1376. [PubMed] [Google Scholar]
- MORENO F.A., GELENBERG A.J., BACHAR K., DELGADO P.L. Pindolol augmentation of treatment-resistant depressed patients. J. Clin. Psychiatry. 1997;58:437–439. doi: 10.4088/jcp.v58n1005. [DOI] [PubMed] [Google Scholar]
- NEWMAN-TANCREDI A., CHAPUT C., GAVAUDAN S., VERRIELE L., MILLAN M.J. Agonist and antagonist actions of (−)-pindolol at recombinant human 5-HT1A receptors. Neuropsychopharmacology. 1998;18:395–398. doi: 10.1016/S0893-133X(97)00169-3. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Co-ordinates. Sydney: Academic Press; 1986. [Google Scholar]
- PEREZ V., GILABERTE I., FAIRES D., ALVAREZ E., ARTIGAS F. Randomised, double blind, placebo-controlled trial of pindolol in combination with fluoxetine antidepressant treatment. Lancet. 1997;349:1594–1597. doi: 10.1016/S0140-6736(96)08007-5. [DOI] [PubMed] [Google Scholar]
- PRISCO S., CAGNOTTO A., TALONE D., DE BLASI A., MENNINI T., ESPOSITO E. Tertatolol, a new β-blocker, is a serotonin (5-hydroxytryptamine1A) receptor antagonist in rat brain. J. Pharmacol. Exp. Ther. 1993;265:739–744. [PubMed] [Google Scholar]
- ROMERO L., BEL N., ARTIGAS F., DE MONTIGNY C., BLIER P. Effect of pindolol at pre- and postsynaptic 5-HT1A receptors: in vivo microdialysis and electrophysiological studies in the rat brain. Neuropsychopharmacol. 1996b;15:349–360. doi: 10.1016/0893-133X(95)00240-E. [DOI] [PubMed] [Google Scholar]
- ROMERO L., HERVAS I., ARTIGAS F. The 5-HT1A antagonist WAY-100635 selectively potentiates the presynaptic effects of serotonergic antidepressants in rat brain. Neurosci. Lett. 1996a;219:123–126. doi: 10.1016/s0304-3940(96)13199-2. [DOI] [PubMed] [Google Scholar]
- SÁNCHEZ C., ARNT J., MOLTZEN E. Assessment of relative efficacies of 5-HT1A receptor ligands by means of in vivo animal models. Eur. J. Pharmacol. 1996a;315:245–254. doi: 10.1016/s0014-2999(96)00621-8. [DOI] [PubMed] [Google Scholar]
- SÁNCHEZ C., ARNT J., MOLTZEN E. The antiaggressive potency of (−)-penbutolol involves both 5-HT1A and 5-HT1B receptors and β-adrenoceptors. Eur. J. Pharmacol. 1996b;297:1–8. doi: 10.1016/0014-2999(95)00727-x. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., WERNER U., HAMON M., GOZLAN H., NICKEL B., SZELENYI I., GÖTHERT M. Anpirtoline, a novel, highly potent 5-HT1B receptor agonist with antinociceptive/antidepressant-like actions in rodents. Br. J. Pharmacol. 1992;105:732–738. doi: 10.1111/j.1476-5381.1992.tb09047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCUVÉE-MOREAU J.J., DRESSE A.E. Effects of various antidepressant drugs on the spontaneous firing rate of locus coeruleus and dorsal raphe neurons of the rat. Eur. J. Pharmacol. 1979;57:219–225. doi: 10.1016/0014-2999(79)90368-6. [DOI] [PubMed] [Google Scholar]
- SHARP T., GARTSIDE S.E., UMBERS V. Effects of co-administration of a monoamine oxidase inhibitor and a 5-HT1A receptor antagonist on 5-hydroxytryptamine cell firing and release. Eur. J. Pharmacol. 1997a;320:15–19. doi: 10.1016/s0014-2999(96)00968-5. [DOI] [PubMed] [Google Scholar]
- SHARP T., UMBERS V., GARTSIDE S.E. Effect of a selective 5-HT reuptake inhibitor in combination with 5-HT1A and 5-HT1B receptor antagonists on extracellular 5-HT in rat frontal cortex in vivo. Br. J. Pharmacol. 1997b;121:941–946. doi: 10.1038/sj.bjp.0701235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP T., ZETTERSTRÖM T.In vivo measurement of monoamine neurotransmitter release using brain microdialysis Monitoring neuronal activity a practical approach 1992Oxford: IRL Press; 147–177.ed. Stamford J.A. pp [Google Scholar]
- SHEARD M.H., ZOLOVICK A., AGHAJANIAN G.K. Raphé neurons: effect of tricyclic antidepressant drugs. Brain Res. 1972;92:291–306. doi: 10.1016/0006-8993(72)90432-5. [DOI] [PubMed] [Google Scholar]
- VANDERMAELEN C.P., BRASELTON J.P. (−)-Penbutolol antagonises 8-OH-DPAT-induced inhibition of rat 5-hydroxytryptaminergic dorsal raphe neurons. Br. J. Pharmacol. 1992;107:2P. [Google Scholar]
- ZANARDI R., ARTIGAS F., FRANCHINI L., SFORZINI L., GASPERINI M., SMERALDI E., PEREZ J. How long should pindolol be associated with paroxetine to improve the antidepressant response. J. Clin. Psychopharmacol. 1997;17:446–450. doi: 10.1097/00004714-199712000-00002. [DOI] [PubMed] [Google Scholar]


