Abstract
The similarity in molecular structure between the histamine H2-agonist dimaprit (3-dimethylamino-propyl-isothiourea) and the endogenous nitric oxide synthase (NOS) substrate L-arginine prompted us to study the effect of dimaprit and some dimaprit analogues on NOS activity. Dimaprit and some of its analogues were tested in an in vitro assay which measures the conversion of [3H]-L-arginine to [3H]-L-citrulline. Dimaprit inhibits rat brain NOS (nNOS) concentration dependently with an IC50 of 49±14 μM.
Removal of one or both of the methyl groups from the non-isothiourea nitrogen of dimaprit improved nNOS inhibitory properties. Aminopropylisothiourea is the most potent compound (IC50=4.1±0.9 μM) of the series followed by methylaminopropylisothiourea (IC50=7.6± μM).
The observed effect of aminopropylisothiourea and methylaminopropyl-isothiourea are probably not due to the compounds themselves but to the corresponding mercaptoalkylguanidines, rearrangement products formed in aqueous solutions. This hypothesis is strengthened by the finding that aminobutylisothiourea is not active since a rearrangement to mercaptobutylguanidine does not occur.
Remarkably, nitrosylation of the isothiourea group of dimaprit decreases nNOS inhibitory activity, while nitrosylation of the guanidine analogue of dimaprit increases the inhibition of nNOS activity.
The pharmacological profile of dimaprit includes inhibition of nNOS. The nNOS inhibitory activity occurs in the same concentration range as the H2-agonist and H3-agonist activity of this compound.
Keywords: Nitric oxide, nitric oxide synthase, inhibition, dimaprit, aminopropylisothiourea, methylaminopropylisothiourea
Introduction
The nitric oxide radical (NO) is involved in various biological processes including endothelium dependent relaxation of blood vessels and lung tissue as well as cell-mediated immunoresponses. NO is also a neurotransmittor associated with learning and memory processes (Schmitt & Walter, 1994). Nitric oxide is synthesized by the nitric oxide synthase enzymes (NOS). These enzymes catalyse the 5 electron oxidation of L-arginine and produce L-citrulline and NO. Three different subtypes of NOS are distinguished. Two constitutive calcium-dependent forms, one discovered in endothelium cells called eNOS, the other present in neuronal cells, known as nNOS (or bNOS from brain). The third subtype is an inducible calcium-independent enzyme, especially present in macrophages, iNOS (Nathan & Xie, 1994). All three subtypes of NOS require heam, calmodulin, flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN) and tetrahydrobiopterin (BH4) as cofactors and NADPH and molecular oxygen as cosubstrates to oxidize L-arginine (Marletta, 1994; Moncada et al., 1991; Nathan & Xie, 1994; Schmitt & Walter, 1994).
The similarity in molecular structure between the histamine H2-agonist, dimaprit (3-dimethylamino-propyl-isothiourea) and the endogenous NOS substrate L-arginine (Table 1) prompted us to study the effect of dimaprit and some dimaprit analogues on NOS activity. Dimaprit and some of its analogues were tested in vitro in a nNOS assay.
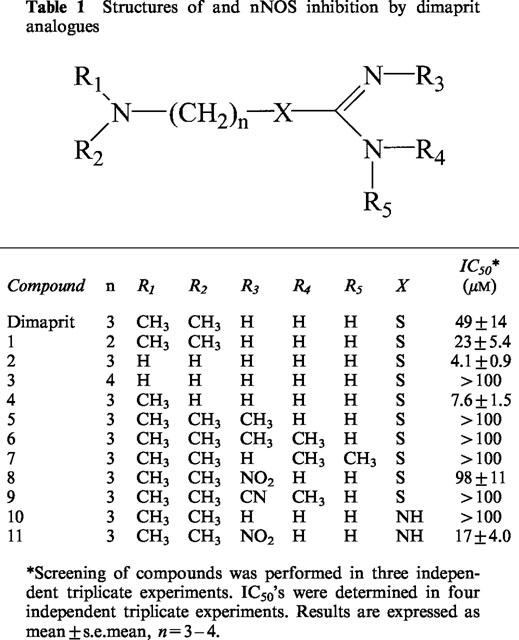
Table 1.
Structures of and nNOS inhibition by dimaprit analogues
Methods
NOS activity
Male Wistar rats (Harlan CPB, 200–220 g) were decapitated and the cerebellum was quickly removed. The isolated cerebellum was suspended in four times its weight of ice cold Tris/HCl-buffer (50 mM, pH 7.4 at 4°C), containing leupeptin (2 μM), phenylmethylsulphonal fluoride 1 mM, dithiothreitol (1 mM), trypsin inhibitor from soybean (10 μg ml−1), aprotinin (2 μg ml−1), EDTA (0.1 mM), sucrose (320 mM), homogenized by pottering and ultra-sonic treatment (three times 10 s). After centrifugation of the homogenate (30 min at 4°C with 100,000×g), the cytosolic fraction was obtained which was used in the experiments.
Forty μl of the cytosolic fraction was added to 60 μl of a 50 mM Tris/HCl buffer (pH 7.4 at 37°C), containing NADPH (1 mM), L-arginine (10 μM), CaCl2 (2 mM), [3H]-L-arginine (6 nCi) and the tested compound in a concentration range from 0.1 μM–0.1 mM. The reaction was carried out for 45 min at 37°C, then terminated by adding 1 ml of ice-cold HEPES buffer (20 mM, pH 5.5 at 4°C) and putting the vials on ice. L-arginine was removed from the sample by adding 1 ml Dowex (Na+ form, 200–400 mesh, pH 7.0) to the mixture. After shaking firmly, the separation was completed by centrifugation (15 min, 400 r.p.m. at 4°C). The [3H]-L-citrulline was measured in 1 ml of the supernantant.
Non-enzymatic conversion was determined by using heated tissue (100°C for 2 min). Inducible NOS activity was determined by using a calcium-free buffer in the presence of EGTA (1 mM) (Bredt & Snyder, 1990). The values found for non-enzymatic conversion and iNOS were subtracted from the total activity to yield specific nNOS activity, expressed in d.p.m. mg−1. It should be noted that non-enzymatic conversion and iNOS activity were very low (<1%) compared with nNOS activity. When inhibitors were used, activity was expressed as enzymatic conversion relative to no inhibitor (100%). The protein content was determined using the Bio-Rad Assay (Bradford, 1976).
Data analysis
The IC50 values of the inhibitors were calculated using linear regression between the data points just above and just below the 50% activity. Calculations were performed using Excel software.
Drugs and materials
Dimaprit and dimaprit analogues used were synthesized according to Sterk et al. (1986). [3H]-L-arginine was obtained from Amersham (Slough, U.K.). All other chemicals used were of the highest purity grade commercially available.
Results
The histamine H2-agonist dimaprit and some of its analogues were tested for nNOS inhibitory activity using an in vitro assay which measures the conversion of [3H]-L-arginine to [3H]-L-citrulline. The NOS inhibitory activity of the compounds was first established at a concentration of 100 μM. Compounds which inhibited NOS by less than 50% at this concentration were considered to be poor NOS inhibitors; for such compounds an IC50 value of >100 μM is listed. Compounds that caused enzyme inhibition greater than 50% at a concentration of 100 μM were tested further to determine the IC50 value (Table 1).
It was found that dimaprit and its analogues caused dose-dependent inhibition of NOS (Figure 1). The IC50 of dimaprit is 49±14 μM. Shortening of the propyl chain of dimaprit to an ethyl chain (compound 1) improved the activity (IC50=23±5.4 μM). Addition of a nitro group or a methyl group (compounds 8 and 5) to the isothiourea moiety resulted in a reduction in inhibitory activity (IC50 98±11 μM and >100 μM respectively). Substitution of hydrogen atoms on both thiourea nitrogens in dimaprit (compound 6, two methyl groups and compound 9, one methyl and one cyanide group) also drastically reduced NOS inhibitory activity (IC50>100 μM for both compounds). The most active compound was aminopropylisothiourea (compound 2, IC50=4.1±0.9 μM) (Southan et al., 1996) while the butyl analogue of compound 2 (compound 3) had a higher IC50 value (IC50>100 μM) than dimaprit.
Figure 1.
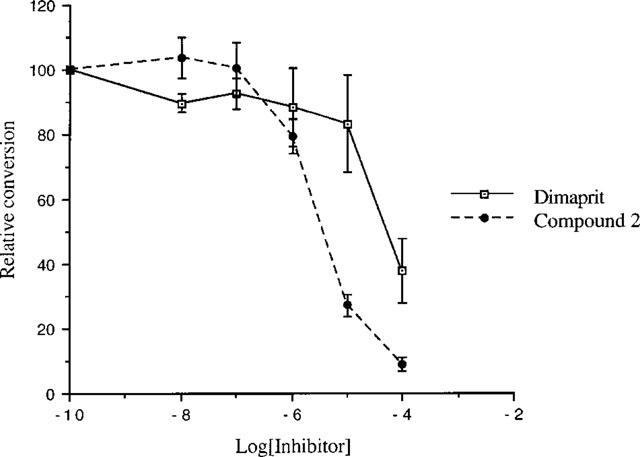
The concentration dependent inhibition of nNOS by the histamine H2-agonist dimaprit and aminopropylisothiourea (compound 2). The activity is expressed as conversion relative to no inhibitor (100%). Results show mean±s.e.mean, n=4.
The guanidine analogue of dimaprit (compound 10) appeared to be a poor NOS inhibitor (IC50>100 μM), while the nitro guanidine derivative (compound 11) had an IC50 of 17±4.0 μM. However, nitrosylation of the isothiourea moiety of dimaprit (compound 8) resulted in an IC50 of 98±11 μM, which was approximately twice that of dimaprit itself.
Discussion
In the present study we have shown that the histamine H2 agonist dimaprit inhibits nNOS. Shortening of the propyl chain to an ethyl chain (compound 1) improves the nNOS inhibitory activity.
Removing the two methyl groups from the non-isothiourea nitrogen also improves the nNOS inhibition properties of the compound when the chain length is three carbons long (compound 2). For the four carbon long butyl analogue, demethylation of the nonisothiourea nitrogen (compound 3) resulted in loss of NOS activity. An explanation for the improved NOS inhibitory activity of the propyl compound (compound 2) might be the reported rearrangement to mercaptoalkylguanidines (Southan et al., 1996). This rearrangement requires a cyclic intermediate that opens to form a mercaptoalkylguanidine. The ring tension of the cyclic intermediate is for five and six rings, derived from the ethyl or the propyl analogue respectively, much smaller than that of the seven ring formed from the butyl analogue. This means that the rearrangement to mercaptoalkylguanidines does not occur as readily for the butyl analogue compared to the ethyl or propyl analogue (Southan et al., 1996; Sterk et al., 1986). If the mercaptoalkylguanidine is the active species, the difference in ring tension in the cyclic intermediate explains the low activity of the demethylated butyl analogue on NOS inhibition (Figure 2).
Figure 2.
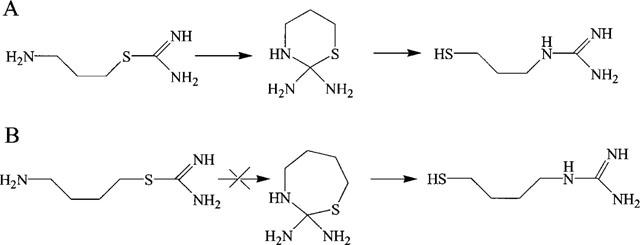
The rearrangement of aminoalkylisothiourea compounds to mercaptoalkylguanidines via a cyclic intermediate. In reaction A the rearrangement of aminopropylisothiourea (compound 2) to mercaptopropylguanidine via a six ring intermediate. Reaction B visualizes the rearrangement of the aminobutylisothiourea (compound 3) to the corresponding mercaptobutylguanidine. This rearrangement does not occur because of the ring-tension in the seven ring intermediate.
Substitutions on either one or both of the isothiourea nitrogens reduce NOS inhibitory activity (compounds 5, 6, 7, 8 and 9). Changing the isothiourea moiety of dimaprit into a guanidine moiety (compound 10) resulted in an enhanced IC50 value. Nitrosylation of the isothiourea moiety (compound 8) rendered the resulting compound less active. This is consistent with the effect of substitution of a hydrogen atom by a methyl group on the isothiourea moiety of dimaprit (compound 5). However, an exception was found for the nitroguanidine compound (compound 11) which has greater nNOS inhibitory activity than the guanidine analogue (compound 10). Nitrosylation affects the pKa of the isothiourea and the guanidine moiety. Normally, these groups are protonated at physiological pH. Due to nitrosylation the groups are almost fully neutral at a pH of 7.4 (Sterk et al., 1984). Surprisingly, nitrosylation had different effects on the nNOS-inhibition, i.e. increasing the activity of the guanidine analogue (compounds 10 vs 11) while reducing the activity of the isothiourea compound (dimaprit vs compound 8). Apparently the guanidine and the isothiourea compound interact differently with nNOS (see also, Southan et al., 1995).
The nNOS inhibition of dimaprit is observed in the same concentration range as that required for H2 and H3 receptor activation (Kohno et al., 1993). To date, no direct coupling of histamine receptors and NOS enzymes has been reported. However, the present data suggest that functional agonism or antagonism may occur. For example, activation of H2 receptors located in the uterus causes relaxation (Parsons et al., 1977) whilst inhibition of NOS will result in a counteracting effect (Papka et al., 1995). Thus the mixed activity of dimaprit has to be taken into account in evaluating the pharmacological effects of this compound. Except for dimaprit itself, none of the tested analogues are active on the histamine H2-receptor (Sterk et al., 1984). Clearly, some of the tested compounds do exhibit a nNOS inhibitory activity. Thus, the structure activity relation for the histamine H2-receptor and the rat nNOS is different, and it may be possible from this series of compounds to develop potent NOS inhibitors without H2 receptor agonist activity.
Acknowledgments
The authors would like to thank Gerard Stender for kindly performing the final nNOS activity experiments and Dr Geert Jan Sterk for providing us with the dimaprit analogues.
Abbreviations
- BH4
tetrahydrobiopterin
- FAD
flavin adenine dinucleotide
- FMN
flavin mononucleotide
- nNOS
neuronal nitric oxide synthase
References
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities protein using the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BREDT D.S., SNYDER S.H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. U.S.A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHNO S., OGAWA K., NABE T., YAMAMURA H., OY K. Dimaprit a histamine H2-agonist inhibits anaphylactic histamine release from mast cells and the decreased release is restored by thioperamide (H3-antagonist) and not by cimetidine (H2-antagonist) Jap. J. Pharmacol. 1993;62:75–79. doi: 10.1254/jjp.62.75. [DOI] [PubMed] [Google Scholar]
- MARLETTA M.A. Nitric Oxide Synthase: Aspects Concerning Structure and Catalysis. Cell. 1994;78:927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric Oxide: Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- NATHAN C., XIE Q. Nitric Oxide Synthases: Roles, Tolls, and Controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- PAPKA R.E., MCNEILL D.L., THOMPSON D., SCHMITT H.H.H.W. Nitric oxide nerves in the uterus are parasympathetic, sensory and contain neuropeptides. Cell Tissue Res. 1995;279:339–349. doi: 10.1007/BF00318490. [DOI] [PubMed] [Google Scholar]
- PARSONS M.E., OWEN D.A.A., GANELLIN C.R., DURANT G.J. Dimaprit - { S - [ 3 - ( N , N - dimethylamino )-propyl ] - isothiourea} – a highly specific histamine H2-receptor agonist. Part 1, Pharmacology. Agents Actions. 1977;7:31–37. doi: 10.1007/BF01964878. [DOI] [PubMed] [Google Scholar]
- SCHMITT H.H.H.W., WALTER U. NO at Work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- SOUTHAN G.J., SZABÓ C., THIEMERMANN C. Isothioureas: potent inhibitors of nitric oxide synthases with variable isoform selectivity. Br. J. Pharmacol. 1995;117:619–632. doi: 10.1111/j.1476-5381.1995.tb13256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUTHAN G.J., ZINGARELLI B., O'CONNOR M., SALZMAN A.L., SZABÓ C. Spontaneous rearrangement of aminoalkylisothioureas into mercaptoalkylguanidines, a novel class of nitric oxide synthase inhibitors with selectivity towards the inducible form. Br. J. Pharmacol. 1996;117:619–632. doi: 10.1111/j.1476-5381.1996.tb15236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERK G.J., VAN DER GOOT H., TIMMERMAN H. Synthesis and histamine H2-activity of dimaprit and some of its analogues. Eur. J. Med. Chem. 1984;19:545–550. [Google Scholar]
- STERK G.J., VAN DER GOOT H., TIMMERMAN H. Synthesis and histamine H2-activity of a series of corresponding histamine and dimaprit analogues. Arch. Pharm. 1986;319:624–630. doi: 10.1002/ardp.19863190710. [DOI] [PubMed] [Google Scholar]


