Figure 1.
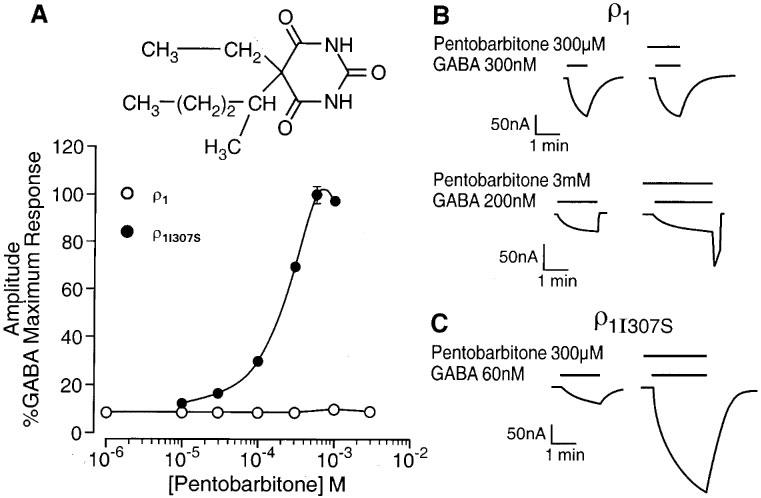
A single transmembrane amino acid governs pentobarbitone sensitivity of the GABA ρ1 receptor. (A) The graph depicts the relationship between the concentration of pentobarbitone (logarithmic scale) and the peak amplitude of the GABA-evoked current (on a linear scale and expressed as percentage of the response to a maximally effective concentration of GABA) at human ρ1 and ρ1I307S GABA receptors. (B) Pentobarbitone (300 μM or 3 mM) has no effect on the GABA (300 or 200 nM respectively)-evoked currents recorded from oocytes expressing human ρ1 receptors. However, upon washout of pentobarbitone 3 mM and GABA 200 nM, a transient inward current (‘rebound') developed. (C) GABA (60 nM)-evoked currents are greatly enhanced by 300 μM pentobarbitone for oocytes expressing human ρ1I307S receptors. The insert shows the chemical structure of pentobarbitone.
