Abstract
The non-selective endothelin (ET) receptor antagonist bosentan has been shown to restore systemic and gut oxygen delivery and reverse intestinal mucosal acidosis in porcine endotoxin shock.
To further elucidate the specific role of the ETA as opposed to the ETB receptor and their effects in the splanchnic region a non-selective (ETMIXra) A-182086 and selective ETA (ETAra) PD155080 and ETB (ETBra) A-192621 receptor antagonists were administered, separately or simultaneously (ETA+Bra) 2 h after onset of endotoxin shock. These four groups were compared to a control group receiving only endotoxin and vehicle.
Thirty-nine pigs were anaesthetized and catheterized for measurement of central and regional haemodynamics. A tonometer in the distal ileum was used for measurement of mucosal PCO2. Blood gases and plasma ET-1-LI levels as well as histological samples from the gut were assessed. Intervention was started 2 h after onset of endotoxemia and the experiments were terminated after 5 h.
Endotoxin-induced changes in systemic, gut oxygen delivery and portal hepatic vascular resistance and systemic acidosis were effectively counteracted by both ETA+Bra and ETMIXra. ETAra administration was not effective while ETBra proved to be fatal as all animals in this group died prior to full time of the experiment. While both ETA+Bra and ETMIXra improved gut oxygen delivery only the latter attenuated the profound endotoxin-induced ileal mucosal acidosis.
The lethal effect seen from selective ETB receptor antagonism in the current study may be due to increased ETA receptor activity as plasma levels of ET-1 is increased several fold by blocking the ETB receptor and thereby the plasma-ET-1-clearing function. Furthermore, a loss of endothelial ETB receptor vasodilating properties may also have contributed to the lethal course in the ETBra group
The findings in this study suggest that ET is involved in the profound endotoxin-induced disturbances in splanchnic homeostasis in porcine endotoxaemia. Furthermore, antagonism of both ETA and ETB receptors is necessary to effectively counteract these changes.
Keywords: Tonometry, septic shock, endotoxin, gut circulation, mucosal damage, endothelin antagonism
Introduction
Septic shock and multiple organ failure remains the leading cause of death in the intensive care unit (Marshall et al., 1995). A large number of inflammatory mediators are involved in the complex pathophysiology of sepsis and endotoxaemia. Several of the manifestations such as pulmonary hypertension, regional hypoperfusion and extravasation of leukocytes can be attributed to endothelin (Oldner et al., 1998; Wanecek et al., 1997). The endothelins are a family of 21 amino acid peptides with powerful vasoconstrictive properties first described in 1988 (Yanagisawa, 1988). Endothelin-1 (ET-1), probably the most important of the endothelins, is mainly produced by the vascular endothelium, acting on three types of receptors. ETA and ETB2 located on vascular smooth muscle cells mediating contraction and ETB1 located on the endothelium mediating vasodilation by release of nitric oxide and prostacyclin (Arai et al., 1994; Pollock & Opgenorth, 1993; De Nucci et al., 1988). Very high plasma levels of ET-1-like immunoreactivity (ET-1-LI) have been demonstrated in various septic conditions and are associated with morbidity and mortality in septic patients (Weitzberg et al., 1991a; Pittet et al., 1991; Takakuwa et al., 1994). A well described feature of septic and endotoxin shock is disturbances in splanchnic perfusion and oxygen delivery/demand balance (Dahn et al., 1987; Ruokonen et al., 1993; Oldner et al., 1998). Hypoperfusion of the gut with concomitant mucosal acidosis followed by reduced barrier function and translocation of gut derived bacteria and endotoxin is a proposed aetiology of multiple organ failure in the critically ill patient (Aranow & Fink, 1996). Infusion of ET-1 in humans reduces gut perfusion (Weitzberg et al., 1991b). Furthermore, ET-1 reduces liver blood flow in a dose dependent manner and has been advocated as an important meditator of liver perfusion disturbances in septic conditions (Bauer et al., 1994; Pannen et al., 1996a). In a previous study from our laboratory administration of bosentan (Ro-47-0203, Hoffman La Roche), a non-selective ET-receptor antagonist, during fulminate endotoxin shock in the pig resulted in a total restoration of a markedly reduced gut oxygen delivery and a reversal of endotoxin-induced intestinal mucosal acidosis (Oldner et al., 1998). The aim of the present study was to further elucidate the specific role of the ETA and ETB receptor, respectively, in gut homeostasis during porcine endotoxaemia. For this purpose selective ETA and ETB as well as a mixed ET receptor antagonist were administered separately or in combination during fulminate endotoxin shock in the pig.
Methods
The experimental protocol for this study was approved by the Ethics Committee for experiments in animals, Stockholm, Sweden.
Anaesthesia and surgical preparation
Thirty-nine landrace pigs of both sexes, weighing 17.3–23.4 kg, were fasted overnight with free access to water. An intramuscular injection of ketamine 20 mg kg−1 and atropine 25 μg kg−1 was used for premedication. Anaesthesia was induced by pentobarbital 12 mg kg−1 intravenously and maintained by a continuous infusion of pentobarbital 3–6 mg kg−1 h−1 and fentanyl 5 μg kg−1 h−1. Anaesthetic level was evaluated prior to administration of muscle relaxants by pain stimuli to the fore hoof with a forceps. Additional doses of pentobarbital or fentanyl were given when needed. Muscle paralysis was achieved by an intravenous infusion of pancurionum bromide 0.5 mg kg−1 h−1. After tracheotomy the animals were mechanically normoventilated with a gas mixture of oxygen in air (fraction of inspiratory O2 0.30) (Servo 900 ventilator, Siemens Elema, Solna, Sweden). The respiratory frequency was set to 18 resp min−1. Body temperature was maintained at 38–39°C. A balloon tipped pulmonary artery catheter was inserted under pressure guidance via a femoral vein to a position in the pulmonary artery. For measurement of arterial blood pressure a catheter was introduced into the abdominal aorta via a femoral artery. A continuous infusion of isotonic saline with glucose 2.5 mg ml−1 at a rate of 20 ml kg−1 h−1 was maintained throughout the experiment. A midline laparotomy was performed. A catheter was introduced into the portal vein. An ultrasonic flow probe (Transonic Systems Inc., Ithaca, NY, U.S.A.) for continuous registration of blood flow was placed around the portal vein. For measurement of intestinal mucosal PCO2 a tonometer (sigmoid catheter, Datex Ohmeda, Helsinki, Finland) was inserted through a small enterotomy in the distal ileum. A catheter was placed in the urinary bladder for collection of urine.
At the end of preparation the abdomen was closed and the animals placed in a left lateral position.
Haemodynamic and blood gas measurements
For continuous measurements and recordings of heart rate (HR) and mean arterial blood pressure (MAP) the arterial catheter was connected to a pressure transducer, while central venous pressure (CVP) and portal venous pressure (PVP) were recorded intermittently on a polygraph (Grass 7B, Quincy, MA, U.S.A.). Cardiac output was measured by thermodilution (Edwards Lab 9520A, St. Ana, CA, U.S.A.) and determined as the mean of a triplicate of 10 ml of ice-cold saline injections and presented as cardiac index (CI, indexed to body weight). Systemic vascular resistance index (SVRI) was calculated as: [(MAP−CVP) Cl−1]. Portal blood flow was recorded continuously on the polygraph and indexed to body weight (Qpvi), presented as ml min−1 kg−1. Gut vascular resistance index (GutVRI, including pancreas and spleen) was calculated as: [(MAP−PVP) Qpvi−1]. The portal venous hepatic vascular resistance index (portal-hepatic VRI) was calculated as: [(PVP−CVP) Qpvi−1]. Blood was collected from the arterial, pulmonary artery and portal venous catheters for analysis of blood gases and acid base status (PO2, PCO2, pH, HCO3− and base excess (BE)) on an ILS 1610 blood gas analyzer (Instrumentation laboratories, Warrington, Cheshire, U.K.). Systemic oxygen delivery index (DO2i) was calculated as: [SaO2×Hb×0.0139×CI] and systemic oxygen consumption index (VO2i) as: [SaO2−mixed venous oxygen saturation (SvO2)×Hb×0.0139×CI]. Gut oxygen delivery index (DO2igut, including pancreas and spleen) was calculated as: [Qpvi×Hb×0.0139×SaO2] and gut oxygen consumption index (VO2igut, including pancreas and spleen) as: [Qpvi×Hb×0.0139×SaO2-portal venous oxygen saturation].
Biochemical analysis
Arterial and portal plasma levels of endothelin-1-like immunoreactivity (ET-1-LI) were analysed with radioimmunoassay as described by Hemsén (Hemsén, 1991). Hb was measured spectrophotometrically (Haemoglobin photometer, LEO, Helsingborg, Sweden).
Endotoxin
Escherichia coli lipopolysaccharide endotoxin (serotype 0111:B4, Sigma, St. Louis, U.S.A.), dissolved in a saline and warmed in order to dissolve any precipitate was used.
Tissue analysis
Biopsies were taken from the distal ileum prior to endotoxaemia (n=8) and in the control (n=6), ETA+Bra (n=5) and ETMIXra (n=4) groups at termination of the experiment. Biopsies were placed on a Millipore filter, fixed in 4% neutral formalin, imbedded in paraffin, sectioned at 4 μ and stained with hematoxilin and eosin. The material was blinded to a pathologist and the histomorphological changes graded in accordance to Chiu et al. (1970). Ileal biopsies were also taken at termination of the experiment from the control (n=5), ETAra (n=5), ETA+Bra (n=5) and ETMIXra (n=4) groups and immediately frozen to −80°C for analysis of myeloperoxidase activity (MPOa). Full-thickness tissue was homogenized and the MPO activity was measured as described previously (Schierwagen et al., 1990).
Experimental protocol
After surgical preparation the animals were allowed 1 h stabilization. An intravenous endotoxin infusion was started at 0 h at a rate of 2.5 μg kg−1 h−1 and increased stepwise during 30 min to reach a final infusion rate of 20 μg kg−1 h−1. The endotoxin infusion was discontinued after 3 h and the animals were observed for another 2 h. After 2 h of endotoxin infusion, eight animals received an intravenous bolus injection of the ETA-receptor antagonist (ETAra) PD155080 (Park Davis, Ann Arbor, MI, U.S.A.) of 10 mg kg−1 dissolved in isotonic saline followed by a continuous infusion of 5 mg kg−1 h−1 for the rest of the experiment. Seven animals received a bolus injection of the ETB-receptor antagonist (ETBra) A-192621 (Abbott Laboratories, Chicago, IL, U.S.A.) of 10 mg kg−1 dissolved in 2% dimethyl sulphoxide (DMSO) followed by a continuous infusion of 5 mg kg−1 h−1 for the rest of the experiment. Six animals received a combination of ETAra and ETBra (ETA+Bra) administered as in the groups above. Eight animals received an intravenous bolus injection of the mixed ET-receptor antagonist (ETMIXra) A-182086 (Abbott Laboratories, IL, U.S.A.) of 5 mg kg−1 dissolved in distilled (deionized) water followed by a continuous infusion of 0.5 mg kg−1 h−1 for the rest of the experiment. Ten animals receiving only endotoxin served as control group. From these controls six received the DMSO vehicle at 2 h while four received a saline vehicle. MAP, HR and portal venous blood flow were monitored continuously. Every 30 min CO was measured, CVP and PVP were recorded and CI, SVRI, GutVRI and portal-hepatic VRI were calculated. Blood samples were collected from the aorta, pulmonary artery and portal vein catheters for analysis of blood gases, in addition Hb and arterial plasma levels of ET-1-LI were analysed hourly. PCO2 in the saline obtained from the tonometer measured every hour was used for calculation of intramucosal mucosal pH (pHi) by means of Henderson-Hasselbalch's equation (pHi=6.1+log [HCO3−]/[PCO2(SS)×0.03] where HCO3− is the bicarbonate level in arterial blood and PCO2(SS) is the PCO2 level at steady state in the saline from the tonometer). Since the saline in the balloon is not fully equilibrated with the luminal PCO2 after 1 h, a correction table, provided by the manufacturer, was used to obtain PCO2 at steady state (PCO2(SS)). Mucosal-arterial PCO2gap was calculated as: [PCO2(SS)−arterial PCO2]. Mucosal-portal PCO2gap was calculated as [PCO2(SS)−portal venous PCO2].
At 5 h the experiments were terminated and the animals were sacrificed by a lethal dose of pentobarbital injected into a central vein.
Statistics
Data are presented as mean (±s.e.mean). An univariate analysis for repeated measures of variance (ANOVA) was used for comparison between groups and changes over time from 0–2 h (prior to intervention) and 2.5 (or 3)–5 h (during intervention) respectively. The time point 2 h was used as covariate for the ANOVA during intervention. All treatment groups were compared to the control group. No statistical analysis was made between the treatment groups. Control animals receiving DMSO vehicle (n=6) were compared to controls receiving saline vehicle (n=4) using ANOVA as described above. Kruskal-Wallis test, if significant, followed by Mann Whitney U-test was used for comparison between control group and other groups for histological grading and MPOa data obtained from biopsies. Differences were considered significant at P<0.05. A computer software program (Statistica 5.1, StatSoft Inc., Tulsa, OK, U.S.A.) was utilized for statistical calculations.
Results
All animals subject to ETBra treatment died from progressive hypodynamic shock prior to termination of experiments. For this reason there were no statistical calculations made between the ETBra animals and the control group during the intervention period. The effect of ETBra treatment is not further commented under results. In addition, one animal in the ETAra group died prior to termination while all other animals survived for full time (5 h) of the experiments.
Systemic haemodynamics, oxygen delivery and consumption (Figure 1 and Table 1)
Figure 1.
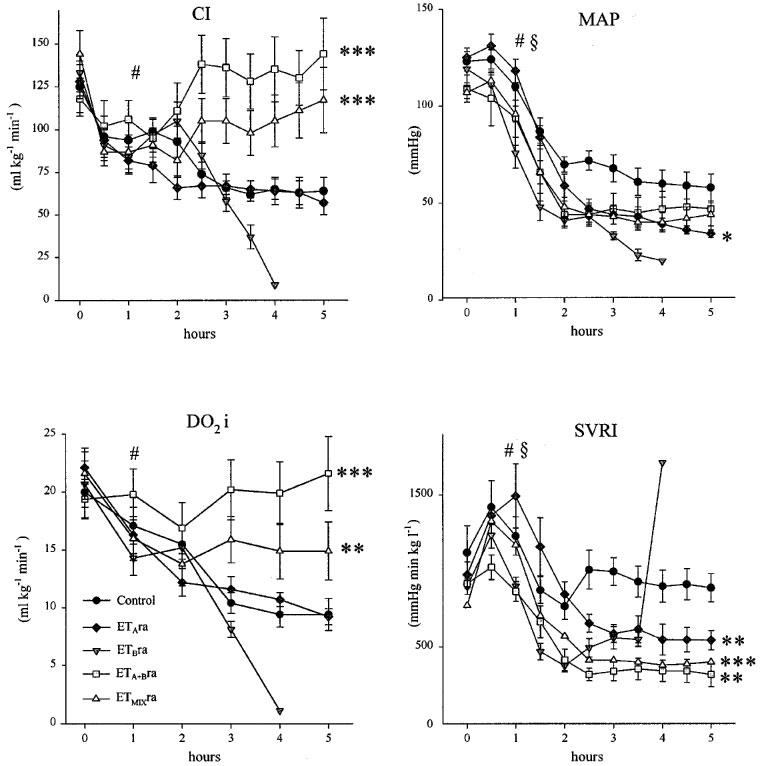
Systemic parameters. Endotoxin control and treated animals. Onset of endotoxin challenge at 0 h and intervention at 2 h. ETAra=ETA receptor antagonist (PD155080); ETBra=ETB receptor antagonist (A-192621); ETA+Bra=simultaneous administration of ETAra and ETBra; and ETMIXra=mixed ET receptor antagonist (A-182086). Data presented as mean (s.e.mean). Comparison between groups performed using ANOVA for repeated measures prior to intervention (0–2 h) and post intervention (2.5 or 3–5 h). Significant changes over time prior to intervention for all groups symbolized by #P<0.01. Significant differences between control group and treated group prior to intervention symbolized by ≈rcub;P<0.05 and during intervention symbolized by *P<0.05, **P<0.01 and ***P<0.001.
Table 1.
Systemic parameters. Endotoxin control and treated animals. Onset of endotoxin challenge at 0 h and intervention at 2 h. Data presented as mean (s.e.mean). Comparison between groups performed using ANOVA for repeated measures prior to intervention (0–2 h) and post intervention (2.5 or 3–5 h)

Endotoxaemia resulted in notable decrease in CI and DO2i that was counteracted by ETA+Bra and ETMIXra treatment while ETAra administration had no effect on these parameters. Endotoxin infusion increased HR significantly while no intervention further affected this parameter. MAP was reduced in response to endotoxin infusion. A significant difference was noted between the control group and the ETBra and ETMIXra group prior to onset of intervention at 2 h. MAP was slightly but significantly reduced by ETAra treatment while no differences were seen between the ETA+Bra and ETMIXra groups and control animals. After an initial peak at aproximately 0.5 h SVRI was mainly unchanged in the control group. A significant difference between ETBra and controls was seen prior to intervention at 2 h. ETAra, ETA+Bra and ETMIXra treatment all reduced SVRI significantly as compared to control animals. VO2i differed significantly between the ETBra, ETMIXra group and control animals prior to onset of intervention. No differences were observed between groups in response to intervention. In accordance with the changes in DO2i the SvO2 was significantly reduced during endotoxaemia. ETA+Bra and ETMIXra treatment resulted in a reversal of this reduction while the decrease was further accentuated in the other groups.
Regional haemodynamics, oxygen delivery and consumption (Figure 2 and Table 2)
Figure 2.

Splanchnic parameters. Endotoxin control and treated animals. Onset of endotoxin challenge at 0 h and intervention at 2 h. ETAra=ETA receptor antagonist (PD155080); ETBra=ETB antagonist receptor (A-192621); ETA+Bra=simultaneous administration of ETAra and ETBra; and ETMIXra=mixed ET receptor antagonist (A-182086). Data presented as mean (s.e.mean). Comparison between groups performed using ANOVA for repeated measures prior to intervention (0–2 h) and post intervention (2.5 or 3–5 h). Significant changes over time prior to intervention for all groups symbolized by #P<0.01. Significant differences between control group and treated group during intervention symbolized by **P<0.01, ***P<0.001.
Table 2.
Splanchnic parameters. Endotoxin control and treated animals. Onset of endotoxin challenge at 0 h and intervention at 2 h. Data presented as mean (s.e.mean). Comparison between groups performed using ANOVA for repeated measures prior to intervention (0–2 h) and post intervention (2.5 or 3–5 h)

Both Qpvi and DO2igut were profoundly reduced by endotoxin challenge. Moreover, these reductions were more pronounced than on the systemic level as the Qpvi Cl−1 ratio was significantly reduced during the initial phase of endotoxaemia. Both ETA+Bra and ETMIXra treatment effectively counteracted the endotoxaemia-induced reduction in Qpvi and DO2igut. ETAra treatment alone had no effect on these parameters. A tendency by ETA+Bra treatment to increase the Qpvi Cl−1 ratio compared to controls did not reach statistical significance (P<0.053). GutVRI increased substantially in response to endotoxaemia with an initial peak at approximately 1 h. A non-significant tendency to reduction was seen in response to ETAra (P<0.07), ETA+Bra (P<0.08) and ETMIXra (P<0.07) treatment. Endotoxin infusion induced an increase in PVP and only ETA+Bra treatment reduced this parameter significantly. A significant difference was seen in VO2igut between ETBra and control animals prior to intervention while no significant differences were observed between groups during intervention. Endotoxin infusion reduced portal venous oxygen saturation markedly. Treatment with ETA+Bra and ETMIXra reversed this reduction effectively. No improvements were seen in the other treatment groups as compared to controls. Portal-hepatic VRI increased notably in response to endotoxin administration. This increase was effectively counteracted by both the ETA+Bra combination and ETMIXra treatment while no changes were seen by ETAra treatment alone.
Intestinal tonometry (Figure 3)
Figure 3.

Tonometrical parameters. Endotoxin control and treated animals. Onset of endotoxin challenge at 0 h and intervention at 2 h. ETAra=ETA receptor antagonist (PD155080); ETBra=ETB receptor antagonist (A-192621); ETA+Bra=simultaneous administration of ETAra and ETBra; and ETMIXra=mixed ET receptor antagonist (A-182086). Data presented as mean (s.e.mean). Comparison between groups performed using ANOVA for repeated measures prior to intervention (0–2 h) and post intervention (3–5 h). Significant changes over time prior to intervention for all groups symbolized by #P<0.05. Significant differences between control group and treated group during intervention symbolized by *P<0.05.
Endotoxin challenge induced profound changes in parameters obtained by tonometry. Intestinal mucosal pHi was notably reduced while increases were seen in both mucosal-arterial PCO2gap and mucosal-portal PCO2gap. These data demonstrate mucosal susceptibility to endotoxin. Despite the clear increase in DO2igut in response to both ETA+Bra and ETMIXra treatment only the latter was able to improve pHi and reduce mucosal-arterial PCO2gap. None of the treatments improved mucosal-portal PCO2gap.
Acid-base status and haemoglobin (Table 1)
Both ETA+Bra and ETMIXra treatment reversed the endotoxin-induced metabolic acidosis seen as reductions in BE and pHa. No beneficial effects were seen from the other treatments. Haemoconcentration with a peak at approximately 1 h was noted during the initial phase of endotoxaemia. Significant differences were seen between the ETAra, ETBra and control group prior to intervention while no significant differences were seen thereafter.
Intestinal histology and myeloperoxidase activity (Figures 4 and 5)
Figure 4.

Box plot of histological intestinal mucosal damage. Grading from 0–5 according to Chiu et al. (1970). Biopsies obtained prior to endotoxaemia (PRE, n=8) first box, control animals (CTRL) at 5 h (n=6) second box, ETA+Bra (simultaneous administration of the ETA receptor antagonist (PD155080) and the ETB receptor antagonist (A-192621)) treated animals at 5 h (n=5) third box and ETMIXra (mixed ET receptor antagonist (A-182086)) treated animals at 5 h (n=4) fourth box. Biopsies obtained prior to endotoxaemia and from treatment groups compared to control animals by Kruskal-Wallis test if significant followed by Mann-Whitney U-test. P values displayed in figure. Data presented as median (line), 25–75% (box), min–max (error bars).
Figure 5.

Box plot of ileal myeloperoxidase activity. Displayed as units per gram protein content. Biopsies obtained from control animals at 5 h (n=5) first box, ETAra (ETA receptor antagonist (PD155080)) treated animals (n=5) at 5 h second box, ETA+Bra (simultaneous administration of the ETA receptor antagonist (PD155080) and the ETB receptor antagonist (A-192621)) treated animals (n=6) at 5 h third box and ETMIXra (mixed ET receptor antagonist (A-182086) treated animals at 5 h (n=4) fourth box. Kruskal-Wallis test for group comparison was not significant. Data presented as median (line), 25–75% (box), min–max (error bars).
Endotoxemia induced clear histological damage to the ileal mucosa where control animals had a significantly higher grade as compared to biopsies obtained prior to endotoxaemia (P<0.003). A tendency for both ETA+Bra and ETMIXra treatment to reduce these histological changes did not reach statistical significance (P<0.09 and 0.12 respectively). No significant differences between groups were observed for MPOa in the ileal biospsies.
Endothelin-1-like immunoreactivity (Figure 6)
Figure 6.

Endothelin-1 like immunoreactivity in arterial plasma. Endotoxin control and treated animals. Onset of endotoxin challenge at 0 h and intervention at 2 h. Data presented as mean (s.e.mean). Comparison between groups performed using ANOVA for repeated measures prior to intervention (0–2 h) and post intervention (3–5 h). Significant changes over time prior to intervention for all groups symbolized by #P<0.05. Significant differences between control group and treated group during intervention symbolized by ***P<0.001.
Endotoxin induced a progressive increase in plasma ET-1-LI in control animals and at 5 h the levels were 4 fold higher than at baseline. The ET-1-LI levels were profoundly increased by administration of ETB receptor antagonists as administration of ETBra, ETA+Bra and ETMIXra at 2 h generated a 10 fold increase compared to baseline levels, significantly higher than the control group. No further increase in plasma ET-1-LI was seen upon ETAra administration.
Saline and DMSO vehicle
No significant differences were observed between control animals receiving saline (n=4) and DMSO (n=6) vehicle respectively in any parameter except for Qpvi which was slightly lower in DMSO vehicle animals (data not shown).
Discussion
A major finding in this study is that simultaneous administration of selective ETA (PD155080) and ETB (A-192621) as well as a non-selective endothelin receptor antagonist (A-182086) significantly increased both systemic and gut oxygen delivery and reversed systemic acidosis. Furthermore, the non-selective ET receptor antagonist reduced ileal mucosal acidosis. In contrast, separate administration of the ETA receptor antagonist provided no major beneficial effects while detrimental effects were noted from selective ETB receptor antagonism alone.
Under physiological conditions ET-1 is constantly produced and participates in regulation of vascular tone (Weitzberg et al., 1994). It acts mostly as a paracrine mediator mainly secreted abluminally suggesting that plasma levels reflect only a fraction of the total activity. However, increased production may be seen in various conditions such as in septic and endotoxin shock (Weitzberg et al., 1991a; Pittet et al., 1991; Takakuwa et al., 1994). During endotoxaemia the increased synthesis and release of endothelin results in several fold increases in plasma levels and ET-1 may then act as a circulating mediator (Ahlborg et al., 1995). Infusion of ET-1 in healthy volunteers induces some of the manifestations seen in sepsis, such as, reduced splanchnic and renal blood flows, lowered cardiac output and pulmonary hypertension (Weitzberg et al., 1991b; 1993). Apart from perfusion disturbances several other features of sepsis and endotoxin shock may be related to endothelin receptor activity. Activation of leukocytes with subsequent extravasation and production of myeloperoxidase and cytokines as well as renal dysfunction have all been demonstrated in response to ET (Lopez Farre et al., 1993; Helset et al., 1994; Rubanyi & Polokoff, 1994; Cunningham, 1997).
In this study CI decreased early in the course of endotoxaemia while MAP decreased somewhat later generating a notable transient increase in SVRI. Administration of ETAra did not affect CI but reduced MAP and SVRI suggesting presence of ETA receptor-mediated vasoconstriction in response to endotoxaemia. Both ETMIXra and ETA+Bra restored CI and reduced SVRI without affecting MAP suggesting that addition of ETB receptor antagonism is important for cardiac output in this model of endotoxaemia. Paradoxically, all animals that received the ETB receptor antagonist alone died prior to full time of the experiment. One important function of the ETB receptor is clearance of plasma ET (Dupuis et al., 1996). Antagonism of the ETB receptor increases circulating ET-1 levels several fold in particular during endotoxaemia (Dupuis et al., 1996; Oldner et al., 1998). Thus, the subsequent increase in ETA receptor stimulation as a consequence of the elevated ET-1 levels may have contributed to the lethal effect of ETB receptor antagonism seen in this study. In addition, loss of endothelial ETB1 receptor-mediated vasodilation modulating the ETA receptor-induced vasoconstriction may also have contributed to the lethal response to selective ETB receptor antagonism. A direct toxic effect by ETBra is not likely as administration simultaneously with ETAra proved to be beneficial. The subtle effects seen from ETAra treatment suggest that ETB receptor activity is of pathophysiological importance in the current model but that the beneficial effects from ETBra treatment may be concealed unless the ETA receptor is antagonized simultaneously. The changes and treatment-response in DO2i did not differ from CI. Surprisingly, despite the marked initial decrease and total restoration of DO2i in two of the treatment groups no corresponding changes were observed in VO2i suggesting a lack of oxygen supply dependency in this model.
Administration of endotoxin notably reduced both Qpvi and DO2igut. This reduction was more pronounced in the gut than in general as the Qpvi Cl−1 ratio decreased significantly during early endotoxin shock. A reduction in gut oxygen delivery is not a mandatory finding during sepsis and endotoxaemia. Increases in gut oxygen delivery often accompanied by even more pronounced increases in gut oxygen consumption are well-described findings in human septic shock (Dahn et al., 1987; Ruokonen et al., 1993). In experimental settings the response is very much dependent on the model utilized. Species, way of shock induction and amount of volume loading may all affect the response. In the current model administration of a non-selective ET-receptor antagonist or simultaneous administration of the selective ETA and ETB receptor antagonists effectively counteracted the endotoxin-induced reduction in Qpvi and DO2igut. In contrast, use of a selective ETA receptor antagonist did not affect these parameters and selective ETB receptor antagonism proved to be fatal. A tendency for ETAra, ETA+Bra and ETMIXra to reduce GutVRI did not reach statistical significance. The finding that non-selective antagonism of ET receptors is effective is consistent with previous studies including a study from our laboratory where, bosentan, a non-selective ET receptor antagonist completely reversed the profound reduction in gut oxygen delivery in a similar experimental model (Wilson et al., 1993; Oldner et al., 1998). The lack of beneficial effects by selective ETA receptor administration, except for a tendency to reduce GutVRI, is somewhat surprising as this receptor has been advocated as an important mediator of splanchnic perfusion disturbances (Massberg et al., 1998; Kayashima et al., 1998). Miura et al. (1996) could demonstrate increased red blood cell velocity and reduced mucosal damage in the ileum when pretreating endotoxaemic rats with BQ123 a selective ETA receptor antagonist. Massberg et al. (1998) effectively counteracted ET-1 induced intestinal mucosal damage in rats pretreated with ETA receptor antagonists while ETB receptor antagonism proved to be non effective.
Mucosal acidosis as seen in the present study is a common feature in septic and endotoxin shock and may have important clinical bearings. Translocation of gut derived endotoxin and bacteria across a hyperpermeable gut barrier have been suggested to promote multiple organ failure in critically ill patients (Deitch, 1990). Reductions in mucosal pHi have in several clinical studies been associated with bad outcome in this group of patients (Doglio et al., 1991; Gutierrez et al., 1992). The use of mucosalarterial PCO2gap, i.e. the difference in carbon dioxide tension between the mucosa as obtained by tonometry and arterial blood has been advocated as a more precise measure of mucosal oxygenation than calculated pHi. Respiratory acid-base changes and infusion of buffering agents may affect calculated pHi without reflecting primary changes in the mucosal oxygenation (Benjamin et al., 1992a,1992b). In the present study both calculated mucosal pHi and mucosal-arterial PCO2gap deteriorated markedly in response to endotoxaemia. This was also the case for mucosal-portal PCO2gap, utilized as a measure of the intestinal mucosa in relation to the gut in general (including the spleen and pancreas). The changes seen reflect mucosal vulnerability during endotoxaemia. Administration of ET antagonists at 2 h had differentiated effects on tonometrical parameters. Only treatment with ETMIXra was able to improve calculated pHi and mucosal-arterial PCO2gap while a tendency by this drug to reduce mucosal-portal PCO2gap did not reach statistical significance. Surprisingly, the combination of ETA+Bra did not improve any of these parameters despite a total restoration of gut oxygen delivery in this group. No difference between ETA+Bra and ETMIXra was seen in the ileal biopsies where both treatments had a tendency, not reaching statistical significance, to improve histological grading that was markedly affected by endotoxaemia. Even though biopsies were obtained from a limited number of animals there was a discrepancy to tonometrical parameters making interpretation of the mucosal effects by ETA+Bra and ETMIXra somewhat difficult. However, in a previous study from our laboratory, administration of bosentan also restored gut oxygen delivery and reduced mucosal acidosis. These differences in findings may be due to different effects of these drugs on the intestinal microcirculation. The balance between ETA and ETB receptor antagonism may be of importance for these effects. A dominant ETA receptor blockade profile has been shown in vitro for bosentan (approximately 20 : 1) and A-182086 (approximately 4 : 1), the drugs with the most beneficial effects on mucosal pHi in the current study (Clozel, 1994). The relation between gut oxygen delivery and mucosal homeostasis is complex in sepsis and endotoxaemia. Increases in regional oxygen consumption and microcirculatory dysregulation may contribute to acidosis in these conditions (Ruokonen et al., 1993; Dahn et al., 1987; Takala, 1997; Humer et al., 1996; Schumacker, 1996). Alterations in blood flow distribution between mucosa and muscularis may generate mucosal acidosis without apparent changes in regional blood flow (Schumacker, 1996). Moreover, disturbances in mitochondrial respiration reducing oxygen utilization capacity during endotoxaemia and sepsis have been suggested to contribute to mucosal acidosis even in the presence of oxygen (Fink, 1997; Unno et al., 1997). ET may also exercise harmful effects on mucosal integrity during non-endotoxaemic conditions. In haemorrhagic shock ET antagonism may protect against shock-induced gastric mucosal ulcerations (Michida et al., 1994; Kitajima et al., 1995) while local administration of ET may induce mucosal damage in non-shocked animals (Whittle & Lopez-Belmonte, 1993; Lopez-Belmonte & Whittle, 1994). Increases in MPOa following activation of neutrophils may be seen in inflammatory states and have been demonstrated in response to ET-1 infusion and in experimental colitis pretreatment with bosentan reduced colonic MPOa in rats (Lopez Farre et al., 1993; Hogaboam et al., 1996). In the current study no reduction of MPOa was seen in response to treatment, findings consistent with a study by Alican et al. where no effect was seen in response to ET antagonism in a model of gut ischaemia-reperfusion in the rat (Alican et al., 1998).
The blood flow through the gut is also dependent on the outflow conditions through the liver (Ayuse et al., 1995). Endotoxaemia-induced increases in PVP, as seen in the current study, may contribute to splanchnic blood pooling and edema formation. Interestingly, regulation of liver perfusion seems to depend largely on ET-receptor activity during endotoxaemia. Infusion of ET-1 into the portal vein markedly reduces sinusoidal blood flow in a dose-dependent manner (Bauer et al., 1994). Release of ET-1 from sinusoidal endothelial cells acting on Ito cells mediating contraction has been one suggested flow regulating mechanism (Tanikawa, 1995). Moreover, endotoxin has been reported to enhance portal venous contractile response to ET-1. Pannen et al. showed that bosentan only slightly reduced hepatic vascular resistance in sham animals while it had a notable effect during endotoxaemia (Pannen et al., 1996b). In the current study endotoxaemia induced a 6 fold increase in portal-hepatic VRI in the control group. Non-selective ET-receptor antagonism effectively counteracted the endotoxaemia-induced increase in this parameter reaching close to baseline levels at 5 h. These findings support the concept of ET being highly important in liver blood flow regulation during endotoxaemia. Both ETA and ETB receptors have been reported to mediate vasoconstrictive response in the liver (Zhang et al., 1995) but the reports in the literature concerning the relative importance of these receptors are not uniform. Partly in agreement with the findings in the present study Iwai et al. noted that hepatocellular damage and blood flow disturbances in perfused rat livers in response to ET-1 infusion were aggravated by both selective ETAra and ETBra while simultaneous administration resulted in improvement of both parameters (Iwai et al., 1998). Likewise Zhang et al. (1997) demonstrated that combined ETA and ETB antagonism was necessary to fully antagonize the effect of ET in isolated perfused rat livers. However, both ETA and ETB receptor antagonism were partly effective in that study. In contrast, Ruetten et al. observed reduced hepatocellular injury in endotoxaemic rats treated with ETB but not ETA receptor antagonists (Ruetten & Thiemermann, 1996) and Nishida et al. (1998) noted an aggravated endotoxin-induced hepatic injury in rats treated with selective ETA receptor antagonists. Thus, the effects of ET on the liver are complex and heterogeneous. Portal-hepatic VRI as calculated in the current study is an estimate of the portal portion of hepatic vascular resistance. Under physiological conditions reductions in portal blood flow is compensated by a decrease in hepatic arterial resistance, an adenosine-dependent mechanism referred to as the hepatic arterial buffer response (Lautt et al., 1985). The changes in portal blood flow and increase in portal-hepatic VRI in the present study may be of particular importance if not compensated for by hepatic arterial blood flow. In endotoxaemia the buffer response has been demonstrated to be impaired (Ayuse et al., 1995). In a recent study by Bathe et al. (1998) the hepatic arterial blood flow was more reduced than portal venous blood flow in endotoxaemic pigs. These data suggest that the changes seen in current study may not have been compensated for by an increase in hepatic arterial blood flow.
A central feature in shock is cellular energy depletion with ATP hydrolysis generating hydrogen ions (Gutierrez & Wulf, 1996). In the current study a profound endotoxin-induced metabolic acidosis was counteracted by both ETA+Bra and ETMIXra. The increase in both systemic and regional oxygen delivery seen in these groups is likely to have contributed to this finding.
The vehicle used for ETBra in the present study, DMSO, may exert anti-oxidant properties. Wray et al. (1998) recently reported reduced liver injury in endotoxaemic rats treated with DMSO. However, no significant differences were seen between DMSO and non-DMSO treated control animals with the exception of Qpvi that was slightly lower in the DMSO group. These data suggest that DMSO did not exert any favourable effects in the present study.
The relative pathophysiological importance of ETA as opposed to ETB receptor activity in sepsis and endotoxaemia is complex. The literature is inconsistent and the findings vary substantially with the experimental model in terms of species, way of shock induction and duration of experiment. Moreover, the results from pretreating as opposed to treatment during fulminate shock as in the current study may differ substantially. The situation may be further complicated by interaction between receptors referred to as receptor ‘cross-talk'. Ozaki et al. (1997) demonstrated reduced ETB receptor affinity for both ETB receptor agonists and antagonists when ETA receptors in the same cell was stimulated. Therefore, data obtained in one model regarding relative importance of subgroups of ET receptors may not prove valid in an other. Clearly, the role of specific ET receptors in human septic pathophysiology must be further elucidated.
In conclusion, in this porcine model administration of endotoxin resulted in profound reductions in both systemic and gut oxygen delivery as well as a notable intestinal mucosal acidosis. Administration of a selective ETA receptor antagonist was not followed by any clear beneficial effects while administration of a selective ETB receptor antagonist proved to be fatal as all animals in this group succumbed prior to termination of the experiment. No animals died in the control group. In contrast, simultaneous administration of the two selective antagonists markedly improved both systemic and gut oxygen delivery and systemic metabolic acidosis as well as reduced portal-hepatic vascular resistance but not mucosal acidosis while administration of the non-selective ET receptor antagonist also reversed mucosal acidosis. The lethal effect seen from selective ETB receptor antagonism alone in the current study may be due to increased ETA receptor activity and concomitant blockade of ETB1 receptor-mediated vasodilation, as plasma levels of ET-1 is increased several fold by blocking the plasma-ET-1-clearing function of the ETB receptor.
The findings in this study suggest that ET is strongly involved in the profound disturbances in splanchnic homeostasis in procine endotoxaemia and that simultaneous antagonism of both ETA and ETB receptors are necessary to counteract these changes.
Acknowledgments
We are grateful to Miss Margareta Stensdotter, Mrs Carina Nilhén and Mrs Karin Rehnqvist for valuable technical assistance. We are also grateful to Dansjö Medical AB, Sweden. This study was supported by the Swedish Medical Research Council (no. 04X-12586), Swedish Society for Medical Research, Laerdal Foundation, Swedish Heart-Lung Foundation, Swedish Society of Medicine, Magn Bergvall Foundation, Åke Wibergs Foundation, The Swedish Foundation for Health Care and Allergy Research and funds from the Karolinska Institute. We thank Abbott Laboratories and Park Davis for the kind gift of drugs A-192621, A-182086 and PD155080, respectively.
Abbreviations
- ANOVA
analysis of variance
- BE
base excess
- CI
cardiac index
- CVP
central venous pressure
- DMSO
dimethyl sulphoxide
- DO2i
systemic oxygen delivery index
- DO2igut
gut oxygen delivery index
- ET
endothelin
- ET-1
endothelin-1
- ETA
endothelin A receptor
- ETA+Bra
simultaneous adminstration of ETAra and ETBra
- ETAra
ETA antagonist (PD155080)
- ET-1-LI
endothelin-1-like immunoreactivity
- ETB
endothelin B receptor
- ETBra
ETB antagonist (A-192621)
- ETMIXra
mixed ET receptor antagonist (A-182086)
- GutVRI
gut vascular resistance index
- Hb
haemoglobin concentration
- HR
heart rate
- MAP
mean arterial blood pressure
- MPOa
myeloperoxidase activity
- PCO2
partial carbon dioxide tension
- pHa
arterial pH
- pHi
intramucosal pH
- PO2
partial oxygen tension
- Portal-hepatic VRI
portal hepatic vascular restistance index
- PVP
portal venous pressure
- Qpvi
portal venous blood flow index
- SaO2
arterial oxygen saturation
- SvO2
mixed venous oxygen saturation
- SVRI
systemic vascular resistance index
- VO2i
systemic oxygen consumption index
- VO2igut
gut oxygen consumption index
References
- AHLBORG G., WIETZBERG E., LUNDBERG J.M. Circulating endothelin-1 reduces splanchnic and renal blood flow and splanchnic glucose production in humans. J. Appl. Physiol. 1995;79:141–145. doi: 10.1152/jappl.1995.79.1.141. [DOI] [PubMed] [Google Scholar]
- ALICAN I., YEGEN C., OLCAY A., KURTEL H., YEGEN B. Ischemia-reperfusion-induced delay in intestinal transit. Role of endothelins. Digestion. 1998;59:343–348. doi: 10.1159/000007513. [DOI] [PubMed] [Google Scholar]
- ARAI H., HORI S., ARAMORI I., OHKUBO H., NAKANISHI S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1994;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- ARANOW J.S., FINK M.P. Determinants of intestinal barrier failure in critical illness. Br. J. Anaesth. 1996;77:71–81. doi: 10.1093/bja/77.1.71. [DOI] [PubMed] [Google Scholar]
- AYUSE T., BRIENZA N., REVELLY J.P., ODONNELL C.P., BOITNOTT J.K., ROBOTHAM J.L. Alterations in liver hemodynamics in an intact porcine model of endotoxin shock. Am. J. Physiol. Heart Circ. Phy. 1995;37:H1106–H1114. doi: 10.1152/ajpheart.1995.268.3.H1106. [DOI] [PubMed] [Google Scholar]
- BATHE O.F., RUDSTON-BROWN B., CHOW A.W.C., PHANG P. Liver as a focus of impaired oxgygenation and cytokine production in a porcine model of endotoxicosis. Crit. Care Med. 1998;26:1698–1706. doi: 10.1097/00003246-199810000-00025. [DOI] [PubMed] [Google Scholar]
- BAUER M., ZHANG J.X., BAUER I., CLEMENS M.G. ET-1 induced alterations of hepatic microcirculation: sinusoidal and extrasinusoidal sites of action. Am. J. Physiol. 1994;267:G143–G149. doi: 10.1152/ajpgi.1994.267.1.G143. [DOI] [PubMed] [Google Scholar]
- BENJAMIN E., HANNON E.M., OROPELLO J.M., STERN P.M., PREMUS G., IBERTI T.J. Effects of respiratory acidosis on gastrointestinal tonometry. Anesthesiology. 1992a;77:A307. [Google Scholar]
- BENJAMIN E., POLOKOFF E., OROPELLO J.M., LEIBOWITZ A.B., IBERTI T.J. Sodium bicarbonate administration affects the diagnostic accuracy of gastrointestinal tonometry in acute mesenteric ischemia. Crit. Care Med. 1992b;20:1181–1183. doi: 10.1097/00003246-199208000-00019. [DOI] [PubMed] [Google Scholar]
- CHIU C.J., MCARDLE A.H., BROWN R., SCOTT H.J., GURD F.N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- CLOZEL M. Pharmacological characterization of bosentan, a new potent orally active non-peptide endothelin receptor antagonist. J. Pharmacol. Exp. Ther. 1994;270:228–235. [PubMed] [Google Scholar]
- CUNNINGHAM M. Endothelin-1 and endothelin-4 stimulate monocyte production of cytokines. Crit. Care Med. 1997;25:958–964. doi: 10.1097/00003246-199706000-00011. [DOI] [PubMed] [Google Scholar]
- DAHN M.S., LANGE P., LOBDELL K., HANS B., JACOBS L.A., MITCHELL R.A. Splanchnic and total body oxygen consumption differences in septic and injured patients. Surgery. 1987;101:69–80. [PubMed] [Google Scholar]
- DEITCH E.A. The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch. Surg. 1990;125:403–404. doi: 10.1001/archsurg.1990.01410150125024. [DOI] [PubMed] [Google Scholar]
- DE NUCCI G., THOMAS R., D'ORLEANS-JUSTE P., ANTUNES E., WALDER C., WARNER T.D., VANE J.R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc. Natl. Acad. Sci. U.S.A. 1988;85:9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOGLIO G.R., PUSAJO J.F., EGURROLA M.A., BONFIGLI G.C., PARRA C., VETERE L., HERNANDEZ M.S., FERNANDEZ S., PALIZAS F., GUTIERREZ G. Gastric mucosal pH as a prognostic index of mortality in critically ill patients. Crit. Care Med. 1991;19:1037–1040. doi: 10.1097/00003246-199108000-00011. [DOI] [PubMed] [Google Scholar]
- DUPUIS J., GORESKY C., FOURNIER A. Pulmonary clearance of circulating endothelin-1 in dogs in vivo: exclusive role of ETB receptors. J. Appl. Physiol. 1996;81:1510–1515. doi: 10.1152/jappl.1996.81.4.1510. [DOI] [PubMed] [Google Scholar]
- FINK M. Cytopathic hypoxia in sepsis. Acta Anaestesiol. Scand. 1997;41:87–95. doi: 10.1111/j.1399-6576.1997.tb05514.x. [DOI] [PubMed] [Google Scholar]
- GUTIERREZ G., PALIZAS F., DOGLIO G., WAINSZTEIN N., GALLESIO A., PACIN J., DUBIN A., SCHIAVI E., JORGE M., PUSAJO J., KLEIN F., SAN ROMAN E., DORFMAN B., SHOTTLENDER J., GINIGER R. Gastric intramucosal pH as a therapeutic index of tissue oxygenation in critically ill patients. Lancet. 1992;339:195–199. doi: 10.1016/0140-6736(92)90002-k. [DOI] [PubMed] [Google Scholar]
- GUTIERREZ G., WULF M.E. Lactic acidosis in sepsis: a commentary. Int. Care Med. 1996;22:6–16. doi: 10.1007/BF01728325. [DOI] [PubMed] [Google Scholar]
- HELSET E., YTREHUS K., TVEITA T., KJAEVE J., JORGENSEN L. ET-1 causes accumulation of leukocytes in the pulmonary circulation. Circ. Shock. 1994;44:201–209. [PubMed] [Google Scholar]
- HEMSÉN A. Biochemical and functional characterization of endothelin peptides with special references to vascular effects. Acta Physiol. Scand. 1991;142:5–61. [PubMed] [Google Scholar]
- HOGABOAM C.M., MULLER M.J., COLLINS S.M., HUNT R.H. An orally active non-selective endothelin receptor antagonist, bosentan, markedly reduces injury in a rat model of colitis. Eur. J. Pharmacol. 1996;309:261–269. doi: 10.1016/0014-2999(96)00276-2. [DOI] [PubMed] [Google Scholar]
- HUMER M.F., PHANG P.T., FRIESEN B.P., ALLARD M.F., GODDARD C.M., WALLEY K.R. Heterogeneity of gut capillary transit times and impaired gut oxygen extraction in endotoxemic pigs. J. Appl. Physiol. 1996;81:895–904. doi: 10.1152/jappl.1996.81.2.895. [DOI] [PubMed] [Google Scholar]
- IWAI M., YAMAUCHI T., SHIMAZU T. Endothelin 1 aggravates acute liver injury in perfused livers of rats after treatment with D-galactosamine. Hepatology. 1998;28:503–509. doi: 10.1002/hep.510280230. [DOI] [PubMed] [Google Scholar]
- KAYASHIMA K., KUDO H., DOI Y., FUJIMOTO S. The role of endothelin-1 in regulation of rat mesenteric microcirculation. J. Cardiovasc. Pharmacol. 1998;31:S126–S127. doi: 10.1097/00005344-199800001-00038. [DOI] [PubMed] [Google Scholar]
- KITAJIMA T., TANI K., YAMAGUCHI T., KUBOTA Y., OKUHIRA M., MIZUNO T., INOUE K. Role of endogenous endothelin in gastric mucosal injury induced by hemorrhagic shock in rats. Digestion. 1995;56:111–116. doi: 10.1159/000201230. [DOI] [PubMed] [Google Scholar]
- LAUTT W.W., LEGARE D.J., D'ALMEIDA M.S. Adenosine as putative regulator of hepatic arterial flow (the buffer response) Am. J. Physiol. 1985;248:H331–H338. doi: 10.1152/ajpheart.1985.248.3.H331. [DOI] [PubMed] [Google Scholar]
- LOPEZ-BELMONTE J., WHITTLE B.J. The involvement of endothelial dysfunction, nitric oxide and prostanoids in the rat gastric microcirculatory responses to endothelin-1. Br. J. Pharmacol. 1994;112:267–271. doi: 10.1111/j.1476-5381.1994.tb13062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPEZ FARRE A., RIESCO A., ESPINOSA G., DIGIUNI E., CERNADAS M.R., ALVAREZ V., MONTON M., RIVAS F., GALLEGO M.J., EGIDO J., CASADO S., CARAMELO C. Effect of endothelin-1 on neutrophil adhesion to endothelial cells and perfused heart. Circulation. 1993;88:1166–1171. doi: 10.1161/01.cir.88.3.1166. [DOI] [PubMed] [Google Scholar]
- MARSHALL J., COOK D., CHRISTOU N.V., BERNARD G.R., SPRUNG C.L., SIBBALD W.J. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit. Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- MASSBERG S., BOROS M., LEIDERER R., BARANYI L., OKADA H., MESSMER K. Endothelin (ET)-1 induced mucosal damage in the rat small intestine: role of ETA receptors. Shock. 1998;9:177–183. doi: 10.1097/00024382-199803000-00004. [DOI] [PubMed] [Google Scholar]
- MICHIDA T., KAWANO S., MASUDA E., KOBAYASHI I., NISHIMURA Y., TSUJII M., HAYASHI N., TAKEI Y., TSUJI S., NAGANO K., FASAMOTO H., KAMADA T. Role of endothelin 1 in hemorrhagic shock-induced gastric mucosal injury in rats. Gastroenterology. 1994;106:988–993. doi: 10.1016/0016-5085(94)90758-7. [DOI] [PubMed] [Google Scholar]
- MIURA S., FUKUMURA D., KUROSE I., HIGUCHI H., KIMURA H., TSUZUKI Y., SHIGEMATSU T., HAN J.Y., TSUCHIYA M., ISHII H. Roles of ET-1 in endotoxin-induced microcirculatory disturbance in rat small intestine. Am. J. Physiol. 1996;271:G461–G469. doi: 10.1152/ajpgi.1996.271.3.G461. [DOI] [PubMed] [Google Scholar]
- NISHIDA T., HUANG T.P., SEIYAMA A., HAMADA E., KAMIIKE W., UESHIMA S., KAZUO H., MATSUDA H. Endothelin A-receptor blockade worsens endotoxin-induced hepatic microcirculatory changes and necrosis. Gastroenterology. 1998;115:412–420. doi: 10.1016/s0016-5085(98)70208-2. [DOI] [PubMed] [Google Scholar]
- OLDNER A., WANECEK M., GOINY M., WEITZBERG E., RUDEHILL A., ALVING K., SOLLEVI A. The endothelin receptor antagonist bosentan restores gut oxygen delivery and reverses intestinal mucosal acidosis in porcine endotoxin shock. Gut. 1998;42:696–702. doi: 10.1136/gut.42.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZAKI S., OHWAKI K., IHARA M., ISHIKAWA K., YANO M. Coexpression studies with endothelin receptor subtypes indicate the existence of intracellular cross-talk between ETA and ETB receptors. J. Biochem. 1997;121:440–447. doi: 10.1093/oxfordjournals.jbchem.a021608. [DOI] [PubMed] [Google Scholar]
- PANNEN B.H., BAUER M., ZHANG J.X., ROBOTHAM J.L., CLEMENS M.G. Endotoxin pretreatment enhances portal venous contractile response to endothelin-1. Am. J. Physiol. 1996a;270:H7–H15. doi: 10.1152/ajpheart.1996.270.1.H7. [DOI] [PubMed] [Google Scholar]
- PANNEN B.H., BAUER M., ZHANG J.X., ROBOTHAM J.L., CLEMENS M.G. A time-dependent balance between endothelins and nitric oxide regulating portal resistance after endotoxin. Am. J. Physiol. 1996b;271:H1953–H1961. doi: 10.1152/ajpheart.1996.271.5.H1953. [DOI] [PubMed] [Google Scholar]
- PITTET J.F., MOREL D.R., HEMSEN A., GUNNING K., LACROIX J.S., SUTER P.M., LUNDBERG J.M. Elevated plasma endothelin-1 concentrations are associated with the severity of illness in patients with sepsis. Ann. Surg. 1991;213:261–264. doi: 10.1097/00000658-199103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK D.M., OPGENORTH T.J. Evidence for endothelin-induced renal vasoconstriction independent of endothelinA receptor activation. Am. J. Physiol. 1993;264:R222. doi: 10.1152/ajpregu.1993.264.1.R222. [DOI] [PubMed] [Google Scholar]
- RUBANYI G.M., POLOKOFF M.A. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol. Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- RUETTEN H., THIEMERMANN C. Effect of selective blockade of endothelin ETB receptors on the liver dysfunction and injury caused by endotoxaemia in the rat. Br. J. Pharmacol. 1996;119:479–486. doi: 10.1111/j.1476-5381.1996.tb15697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUOKONEN E., TAKALA J., KARI A., SAXEN H., MERTSOLA J., HANSEN E.J. Regional blood flow and oxygen transport in septic shock. Crit. Care Med. 1993;21:1296–1303. doi: 10.1097/00003246-199309000-00011. [DOI] [PubMed] [Google Scholar]
- SCHIERWAGEN C., BYLUND-FELLENIUS A.-C., LUNDBERG C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J. Pharmacol. Meth. 1990;23:179–186. doi: 10.1016/0160-5402(90)90061-o. [DOI] [PubMed] [Google Scholar]
- SCHUMACKER P.Regulation of gut oxygen delivery, cellular oxygen supply and metabolic activity Gut dysfunction in critical illness 199626Springer: Berlin; 65–75.In: Rombeau, J.L., Takala, J. (eds) [Google Scholar]
- TAKAKUWA T., ENDO S., NAKAE H., KIKICHI M., SUZUKI T., INADA K., YOSHIDA M. Plasma levels of TNF-alpha, endothelin-1 and thrombomodulin in patients with sepsis. Res. Comm. Chem. Pathol. Pharmacol. 1994;84:261–269. [PubMed] [Google Scholar]
- TAKALA J. Regional contribution to hypermetabolism following trauma. Baillieres Clin. Endocrinol. Metab. 1997;11:617–627. doi: 10.1016/s0950-351x(97)80894-4. [DOI] [PubMed] [Google Scholar]
- TANIKAWA K. Hepatic sinusoidal cells and sinusoidal circulation. J. Gastroenterol. Hepatol. 1995;10:S8–S11. doi: 10.1111/j.1440-1746.1995.tb01806.x. [DOI] [PubMed] [Google Scholar]
- UNNO N., WANG H., MENCONI M.J., TYGTAT S.H.A.J., LARKIN V., SMITH M., MORIN M.J., CHAVEZ A., HODIN R.A., FINK M.P. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology. 1997;113:1246–1257. doi: 10.1053/gast.1997.v113.pm9322519. [DOI] [PubMed] [Google Scholar]
- WANECEK M., OLDNER A., RUDEHILL A., SOLLEVI A., ALVING K., WEITZBERG E. Cardiopulmonary dysfunction during porcine endotoxin shock is effectively counteracted by the endothelin receptor antagonist bosentan. Shock. 1997;7:364–370. doi: 10.1097/00024382-199705000-00009. [DOI] [PubMed] [Google Scholar]
- WEITZBERG E., AHLBORG G., LUNDBERG J.M. Long-lasting vasoconstriction and efficient regional extraction of endothelin-1 in human splanchnic and renal tissues. Biochem. Biophys. Res. Comm. 1991b;180:1298–1303. doi: 10.1016/s0006-291x(05)81336-1. [DOI] [PubMed] [Google Scholar]
- WEITZBERG E., AHLBORG G., LUNDBERG J.M. Differences in vascular effects and removal of endothelin-1 in human lung, brain, and skeletal muscle. Clin. Physiol. 1993;13:653–662. doi: 10.1111/j.1475-097x.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- WEITZBERG E., LUNDBERG J.M., RUDEHILL A. Elevated plasma levels of endothelin in patients with sepsis syndrome. Circ. Shock. 1991a;33:222–227. [PubMed] [Google Scholar]
- WEITZBERG E., RUDEHILL A., MODIN A., LUNDBERG J.M. Porcine intrinsic pulmonary and systemic vascular tone is endothelin-dependent. Acta Physiol. Scand. 1994;152:433–434. doi: 10.1111/j.1748-1716.1994.tb09827.x. [DOI] [PubMed] [Google Scholar]
- WHITTLE B.J., LOPEZ-BELMONTE J. Actions and interactions of endothelins, prostacyclin and nitric oxide in the gastric mucosa. J. Physiol. Pharmacol. 1993;44:91–107. [PubMed] [Google Scholar]
- WILSON M.A., STEEB G.D., GARRISON R.N. Endothelins mediate intestinal hypoperfusion during bacteremia. J. Surg. Res. 1993;55:168–175. doi: 10.1006/jsre.1993.1125. [DOI] [PubMed] [Google Scholar]
- WRAY G.M., HINDS C.J., THIEMERMANN C. Effects of inhibitors of poly (ADP-ribose) synthase activity on hypotension and multiple organ dysfunction caused by endotoxin. Shock. 1998;10:13–19. doi: 10.1097/00024382-199807000-00003. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA M. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- ZHANG B., CALMUS Y., WEN L., SOGNI P., LOTERSZTAJN S., HOUSSIN D., WEILL B. Endothelin-1 induces liver vasoconstriction through both ETA and ETB receptors. J. Hepatol. 1997;26:1104–1110. doi: 10.1016/s0168-8278(97)80119-5. [DOI] [PubMed] [Google Scholar]
- ZHANG J.X., BAUER M., CLEMENS M.G. Vessel and target cell specific actions of endothelin-1 and endothelin-3 in rat liver. Am. J. Physiol. 1995;269:G269–G277. doi: 10.1152/ajpgi.1995.269.2.G269. [DOI] [PubMed] [Google Scholar]


