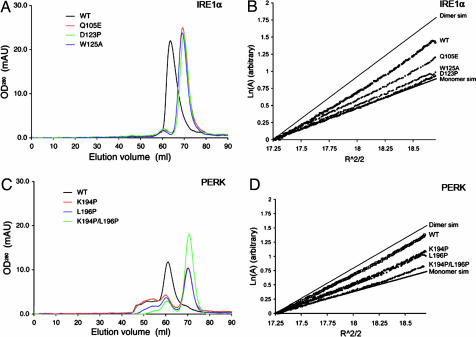
Fig. 3.
The luminal domains of IRE1 and PERK share a similar dimerization mechanism. (A and C) Gel-filtration analysis. WT human IRE1α NLD eluted as a 158-kDa protein upon gel filtration (black line), and mutants Q105E (red line), D123P (green line), and W125A (blue line) eluted 6 ml later (A). WT murine PERK NLD eluted as a 181-kDa protein (black line), and mutants K194P (red line), L196P (blue line), and K194P/L196P (green line) eluted 10 ml later (C). (B and D) Analytical ultracentrifugation sedimentation equilibrium analysis. Point mutations in human IRE1α NLD (B) and murine PERK NLD (D) shift the dimer/monomer equilibrium toward the monomeric species. The IRE1α NLD mutant D123P and the PERK NLD double mutant K194P/L196P were exclusively monomeric.