Figure 5.
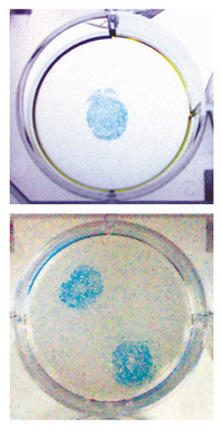
Regio-specific transduction of target cells on a solid surface by immobilized adenoviral vectors. Six-well cell culture plates, coated with poly-D-lysine (well diameter, 3.5 cm; Biocoat Poly-D-Lysine Cellware 6-Well Plates), were used as the solid surface. First, spots of biotin moieties on the wells were created by using sulfo-NHS-LC-biotin. Plastic rings (inner diameter, 1.4 cm) were used to specify biotinylation areas, and the remaining area of the wells was covered with a layer of 2% agarose. These spot areas within plastic rings were treated with 1 mg/ml sulfo-NHS-LC-biotin in PBS (pH 7.4) (50 μl per spot) for 2 h at 4°C, followed by the remove of unreacted biotinylation reagent. Neutralite avidin (25 μg in 50 μl PBST per spot) was added to the spot areas within the plastic rings and allowed to bind to biotin moieties of the well surface. Unbound Neutralite avidin was removed, and biotinylated Ad5.CMV-LacZ (1 × 107 viral particles per spot), prepared by treatment with 15 μg/ml sulfo-NHS-LC-biotin, was incubated in the spot areas within plastic rings at 25°C for 2 h, followed by the removal of unbound viral particles. The plastic rings and agarose were removed from the wells, which were then washed with PBS. D-17 cells (2.5 × 105 per well) were applied to the entirety of the wells, cultured at 37°C for 48 h, and stained for the expression of the lacZ gene.
