Abstract
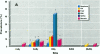
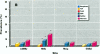
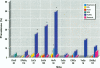
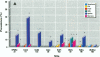
Relationships between hepatic lesions and chemical contaminant concentrations in sediments, stomach contents, liver tissue, and bile were statistically evaluated in three species of bottomfish, English sole (Pleuronectes vetulus), starry flounder (Platichthys stellatus), and white croaker (Genyonemus lineatus), captured from 27 urban and nonurban sites on the Pacific Coast from Alaska to southern California. Lesions detected were neoplasms, preneoplastic foci of cellular alteration, nonneoplastic proliferative lesions, unique or specific degenerative/necrotic lesions, nonspecific degenerative/necrotic lesions, and hydropic vacuolation of biliary epithelial cells and hepatocytes. In general, lesion prevalences were significantly higher in all three species captured at chemically contaminated urban sites, and certain lesions had significantly higher relative risks of occurrence at urban sites in Puget Sound, San Francisco Bay, the vicinity of Los Angeles, and San Diego Bay. Concentrations of polycyclic aromatic hydrocarbons, polychlorinated biphenyls, DDT and its derivatives, and chlordanes and dieldrin in sediment, stomach contents, liver, and fluorescent aromatic compounds in bile were significant risk factors for the occurrence of neoplastic, preneoplastic, nonneoplastic proliferative, and specific degenerative/necrotic lesions, as well as hydropic vacuolation. Fish age also had a significant influence on occurrence of several hepatic lesions, but gender was rarely a significant risk factor. These relationships provide strong evidence for the involvement of environmental contaminants in the etiology of hepatic lesions in several marine bottomfish species and clearly indicate the utility of these lesions as biomarkers of contaminant-induced effects in wild fish.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P. C., Harshbarger J. C., Hartman K. J. Relationship between liver tumors and age in brown bullhead populations from two Lake Erie tributaries. Sci Total Environ. 1990 May 1;94(1-2):71–87. doi: 10.1016/0048-9697(90)90365-2. [DOI] [PubMed] [Google Scholar]
- Bodammer J. E., Murchelano R. A. Cytological study of vacuolated cells and other aberrant hepatocytes in winter flounder from Boston Harbor. Cancer Res. 1990 Oct 15;50(20):6744–6756. [PubMed] [Google Scholar]
- Bunton T. E. Hepatopathology of diethylnitrosamine in the medaka (Oryzias latipes) following short-term exposure. Toxicol Pathol. 1990;18(2):313–323. doi: 10.1177/019262339001800210. [DOI] [PubMed] [Google Scholar]
- Collier T. K., Singh S. V., Awasthi Y. C., Varanasi U. Hepatic xenobiotic metabolizing enzymes in two species of benthic fish showing different prevalences of contaminant-associated liver neoplasms. Toxicol Appl Pharmacol. 1992 Apr;113(2):319–324. doi: 10.1016/0041-008x(92)90131-b. [DOI] [PubMed] [Google Scholar]
- Columbano A., Rajalakshmi S., Sarma D. S. Requirement of cell proliferation for the initiation of liver carcinogenesis as assayed by three different procedures. Cancer Res. 1981 Jun;41(6):2079–2083. [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Farber E., Sarma D. S. Hepatocarcinogenesis: a dynamic cellular perspective. Lab Invest. 1987 Jan;56(1):4–22. [PubMed] [Google Scholar]
- Fong A. T., Hendricks J. D., Dashwood R. H., Van Winkle S., Lee B. C., Bailey G. S. Modulation of diethylnitrosamine-induced hepatocarcinogenesis and O6-ethylguanine formation in rainbow trout by indole-3-carbinol, beta-naphthoflavone, and Aroclor 1254. Toxicol Appl Pharmacol. 1988 Oct;96(1):93–100. doi: 10.1016/0041-008x(88)90251-7. [DOI] [PubMed] [Google Scholar]
- Foulkes E. C. The concept of critical levels of toxic heavy metals in target tissues. Crit Rev Toxicol. 1990;20(5):327–339. doi: 10.3109/10408449009089868. [DOI] [PubMed] [Google Scholar]
- Frith C. H., Ward J. M. A morphologic classification of proliferative and neoplastic hepatic lesions in mice. J Environ Pathol Toxicol. 1979 Dec;3(1-2):329–351. [PubMed] [Google Scholar]
- Harshbarger J. C., Clark J. B. Epizootiology of neoplasms in bony fish of North America. Sci Total Environ. 1990 May 1;94(1-2):1–32. doi: 10.1016/0048-9697(90)90362-x. [DOI] [PubMed] [Google Scholar]
- Hawkins W. E., Walker W. W., Overstreet R. M., Lytle J. S., Lytle T. F. Carcinogenic effects of some polycyclic aromatic hydrocarbons on the Japanese medaka and guppy in waterborne exposures. Sci Total Environ. 1990 May 1;94(1-2):155–167. doi: 10.1016/0048-9697(90)90370-a. [DOI] [PubMed] [Google Scholar]
- Hayes M. A., Safe S. H., Armstrong D., Cameron R. G. Influence of cell proliferation on initiating activity of pure polychlorinated biphenyls and complex mixtures in resistant hepatocyte in vivo assays for carcinogenicity. J Natl Cancer Inst. 1985 May;74(5):1037–1041. [PubMed] [Google Scholar]
- Hayes M. A., Smith I. R., Rushmore T. H., Crane T. L., Thorn C., Kocal T. E., Ferguson H. W. Pathogenesis of skin and liver neoplasms in white suckers from industrially polluted areas in Lake Ontario. Sci Total Environ. 1990 May 1;94(1-2):105–123. doi: 10.1016/0048-9697(90)90367-4. [DOI] [PubMed] [Google Scholar]
- Hendricks J. D., Meyers T. R., Shelton D. W., Casteel J. L., Bailey G. S. Hepatocarcinogenicity of benzo[a]pyrene to rainbow trout by dietary exposure and intraperitoneal injection. J Natl Cancer Inst. 1985 Apr;74(4):839–851. [PubMed] [Google Scholar]
- Hendricks J. D., Meyers T. R., Shelton D. W. Histological progression of hepatic neoplasia in rainbow trout (Salmo gairdneri). Natl Cancer Inst Monogr. 1984 May;65:321–336. [PubMed] [Google Scholar]
- Hendricks J. D., Putnam T. P., Sinnhuber R. O. Effect of dietary dieldrin on aflatoxin B1 carcinogenesis in rainbow trout (Salmo gairdneri). J Environ Pathol Toxicol. 1979 Jan-Feb;2(3):719–728. [PubMed] [Google Scholar]
- Hodge H. C., Boyce A. M., Deichmann W. B., Kraybill H. F. Toxicology and no-effect levels of aldrin and dieldrin. Toxicol Appl Pharmacol. 1967 Mar;10(3):613–675. doi: 10.1016/0041-008x(67)90100-7. [DOI] [PubMed] [Google Scholar]
- Kimbrough R. D., Linder R. E., Gaines T. B. Morphological changes in livers of rats fed polychlorinated biphenyls: light microscopy and ultrastructure. Arch Environ Health. 1972 Nov;25(5):354–364. doi: 10.1080/00039896.1972.10666186. [DOI] [PubMed] [Google Scholar]
- Kimbrough R. D., Linder R. E. Induction of adenofibrosis and hepatomas of the liver in BALB-cJ mice by polychlorinated biphenyls (Aroclor 1254). J Natl Cancer Inst. 1974 Aug;53(2):547–552. doi: 10.1093/jnci/53.2.547. [DOI] [PubMed] [Google Scholar]
- Koller L. D., Zinkl J. G. Pathology of polychlorinated biphenyls in rabbits. Am J Pathol. 1973 Mar;70(3):363–378. [PMC free article] [PubMed] [Google Scholar]
- Koza R. A., Moore M. J., Stegeman J. J. Elevated ornithine decarboxylase activity, polyamines and cell proliferation in neoplastic and vacuolated liver cells of winter flounder (Pleuronectes americanus) Carcinogenesis. 1993 Mar;14(3):399–405. doi: 10.1093/carcin/14.3.399. [DOI] [PubMed] [Google Scholar]
- Krahn M. M., Burrows D. G., MacLeod W. D., Jr, Malins D. C. Determination of individual metabolites of aromatic compounds in hydrolyzed bile of English sole (Parophrys vetulus) from polluted sites in Puget Sound, Washington. Arch Environ Contam Toxicol. 1987 Sep;16(5):511–522. doi: 10.1007/BF01055807. [DOI] [PubMed] [Google Scholar]
- Krahn M. M., Rhodes L. D., Myers M. S., Moore L. K., MacLeod W. D., Jr, Malins D. C. Associations between metabolites of aromatic compounds in bile and the occurrence of hepatic lesions in English sole (Parophrys vetulus) from Puget Sound, Washington. Arch Environ Contam Toxicol. 1986 Jan;15(1):61–67. doi: 10.1007/BF01055249. [DOI] [PubMed] [Google Scholar]
- Landahl J. T., McCain B. B., Myers M. S., Rhodes L. D., Brown D. W. Consistent associations between hepatic lesions in English sole (Parophrys vetulus) and polycyclic aromatic hydrocarbons in bottom sediment. Environ Health Perspect. 1990 Nov;89:195–203. doi: 10.1289/ehp.9089195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHUR D. S. Studies on the histopathological changes induced by DDT in the liver, kidney, and intestine of certain fishes. Experientia. 1962 Nov 15;18:506–509. doi: 10.1007/BF02151600. [DOI] [PubMed] [Google Scholar]
- Malins D. C., McCain B. B., Myers M. S., Brown D. W., Krahn M. M., Roubal W. T., Schiewe M. H., Landahl J. T., Chan S. L. Field and laboratory studies of the etiology of liver neoplasms in marine fish from Puget Sound. Environ Health Perspect. 1987 Apr;71:5–16. doi: 10.1289/ehp.87715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maronpot R. R., Montgomery C. A., Jr, Boorman G. A., McConnell E. E. National Toxicology Program nomenclature for hepatoproliferative lesions of rats. Toxicol Pathol. 1986;14(2):263–273. doi: 10.1177/019262338601400217. [DOI] [PubMed] [Google Scholar]
- Metcalfe C. D., Balch G. C., Cairns V. W., Fitzsimons J. D., Dunn B. P. Carcinogenic and genotoxic activity of extracts from contaminated sediments in western Lake Ontario. Sci Total Environ. 1990 May 1;94(1-2):125–141. doi: 10.1016/0048-9697(90)90368-5. [DOI] [PubMed] [Google Scholar]
- Murchelano R. A., Wolke R. E. Neoplasms and nonneoplastic liver lesions in winter flounder, Pseudopleuronectes americanus, from Boston Harbor, Massachusetts. Environ Health Perspect. 1991 Jan;90:17–26. doi: 10.1289/ehp.90-1519495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. S., Landahl J. T., Krahn M. M., Johnson L. L., McCain B. B. Overview of studies on liver carcinogenesis in English sole from Puget Sound; evidence for a xenobiotic chemical etiology. I: Pathology and epizootiology. Sci Total Environ. 1990 May 1;94(1-2):33–50. doi: 10.1016/0048-9697(90)90363-y. [DOI] [PubMed] [Google Scholar]
- Myers M. S., Landahl J. T., Krahn M. M., McCain B. B. Relationships between hepatic neoplasms and related lesions and exposure to toxic chemicals in marine fish from the U.S. West Coast. Environ Health Perspect. 1991 Jan;90:7–15. doi: 10.1289/ehp.90-1519518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. S., Rhodes L. D., McCain B. B. Pathologic anatomy and patterns of occurrence of hepatic neoplasms, putative preneoplastic lesions, and other idiopathic hepatic conditions in English sole (Parophrys vetulus) from Puget Sound, Washington. J Natl Cancer Inst. 1987 Feb;78(2):333–363. [PubMed] [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): biochemistry, toxicology, and mechanism of action. Crit Rev Toxicol. 1984;13(4):319–395. doi: 10.3109/10408448409023762. [DOI] [PubMed] [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs): mutagenicity and carcinogenicity. Mutat Res. 1989 Jan;220(1):31–47. doi: 10.1016/0165-1110(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Schultz R. J., Schultz M. E. Characteristics of a fish colony of Poeciliopsis and its use in carcinogenicity studies with 7,12-dimethylbenz[a]anthracene and diethylnitrosamine. Natl Cancer Inst Monogr. 1984 May;65:5–13. [PubMed] [Google Scholar]
- Sell S. Is there a liver stem cell? Cancer Res. 1990 Jul 1;50(13):3811–3815. [PubMed] [Google Scholar]
- Shelton D. W., Goeger D. E., Hendricks J. D., Bailey G. S. Mechanisms of anti-carcinogenesis: the distribution and metabolism of aflatoxin B1 in rainbow trout fed aroclor 1254. Carcinogenesis. 1986 Jul;7(7):1065–1071. doi: 10.1093/carcin/7.7.1065. [DOI] [PubMed] [Google Scholar]
- Shelton D. W., Hendricks J. D., Bailey G. S. The hepatocarcinogenicity of diethylnitrosamine to rainbow trout and its enhancement by Aroclors 1242 and 1254. Toxicol Lett. 1984 Jul;22(1):27–31. doi: 10.1016/0378-4274(84)90041-9. [DOI] [PubMed] [Google Scholar]
- Silberhorn E. M., Glauert H. P., Robertson L. W. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol. 1990;20(6):440–496. doi: 10.3109/10408449009029331. [DOI] [PubMed] [Google Scholar]
- Stein J. E., Reichert W. L., French B., Varanasi U. 32P-postlabeling analysis of DNA adduct formation and persistence in English sole (Pleuronectes vetulus) exposed to benzo[a]pyrene and 7H-dibenzo[c,g]carbazole. Chem Biol Interact. 1993 Jul;88(1):55–69. doi: 10.1016/0009-2797(93)90084-c. [DOI] [PubMed] [Google Scholar]
- Varanasi U., Nishimoto M., Reichert W. L., Le Eberhart B. T. Comparative metabolism of benzo(a)pyrene and covalent binding to hepatic DNA in English sole, starry flounder, and rat. Cancer Res. 1986 Aug;46(8):3817–3824. [PubMed] [Google Scholar]
- Vogelbein W. K., Fournie J. W., Van Veld P. A., Huggett R. J. Hepatic neoplasms in the mummichog Fundulus heteroclitus from a creosote-contaminated site. Cancer Res. 1990 Sep 15;50(18):5978–5986. [PubMed] [Google Scholar]
- Williams G. M. The significance of preneoplastic liver lesions in experimental animals. Adv Vet Sci Comp Med. 1987;31:21–44. doi: 10.1016/b978-0-12-039231-5.50007-3. [DOI] [PubMed] [Google Scholar]
- Zerban H., Rabes H. M., Bannasch P. Sequential changes in growth kinetics and cellular phenotype during hepatocarcinogenesis. J Cancer Res Clin Oncol. 1989;115(4):329–334. doi: 10.1007/BF00400958. [DOI] [PubMed] [Google Scholar]
- Zinkl J. G. Skin and liver lesions in rats fed a polychlorinated biphenyl mixture. Arch Environ Contam Toxicol. 1977;5(2):217–228. doi: 10.1007/BF02220905. [DOI] [PubMed] [Google Scholar]