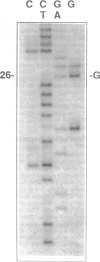
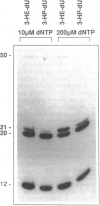
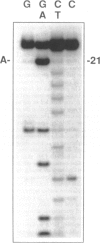
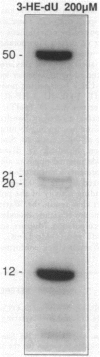
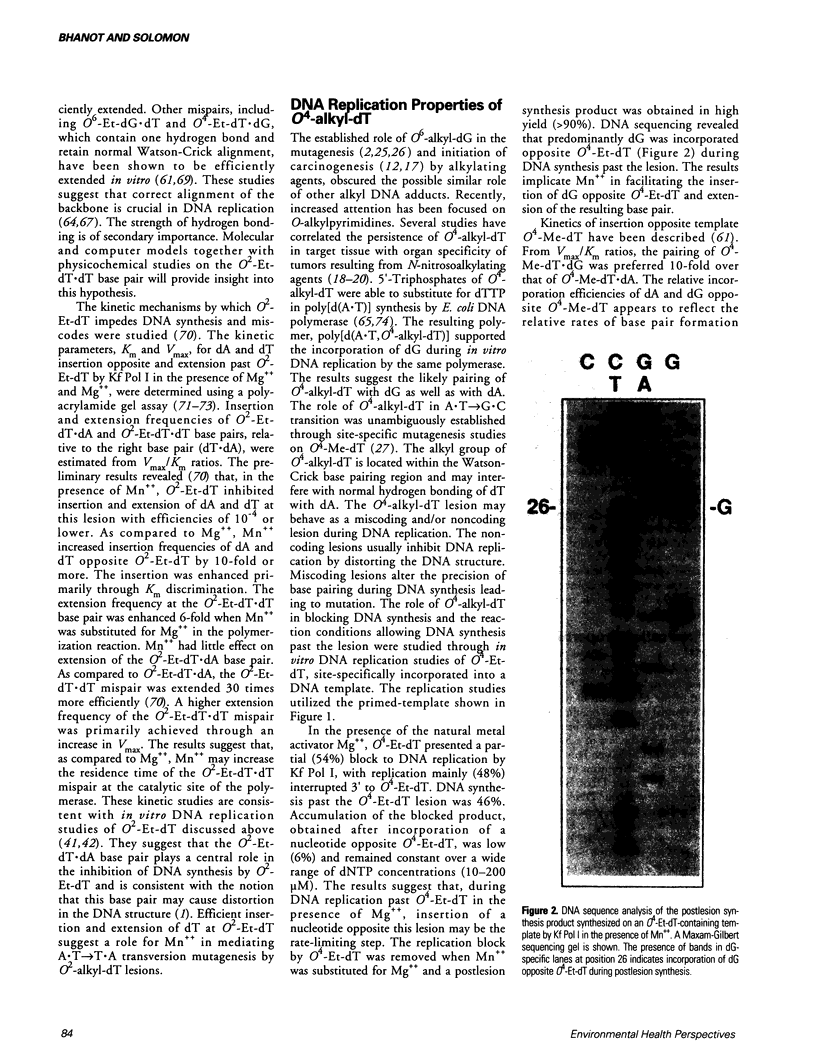
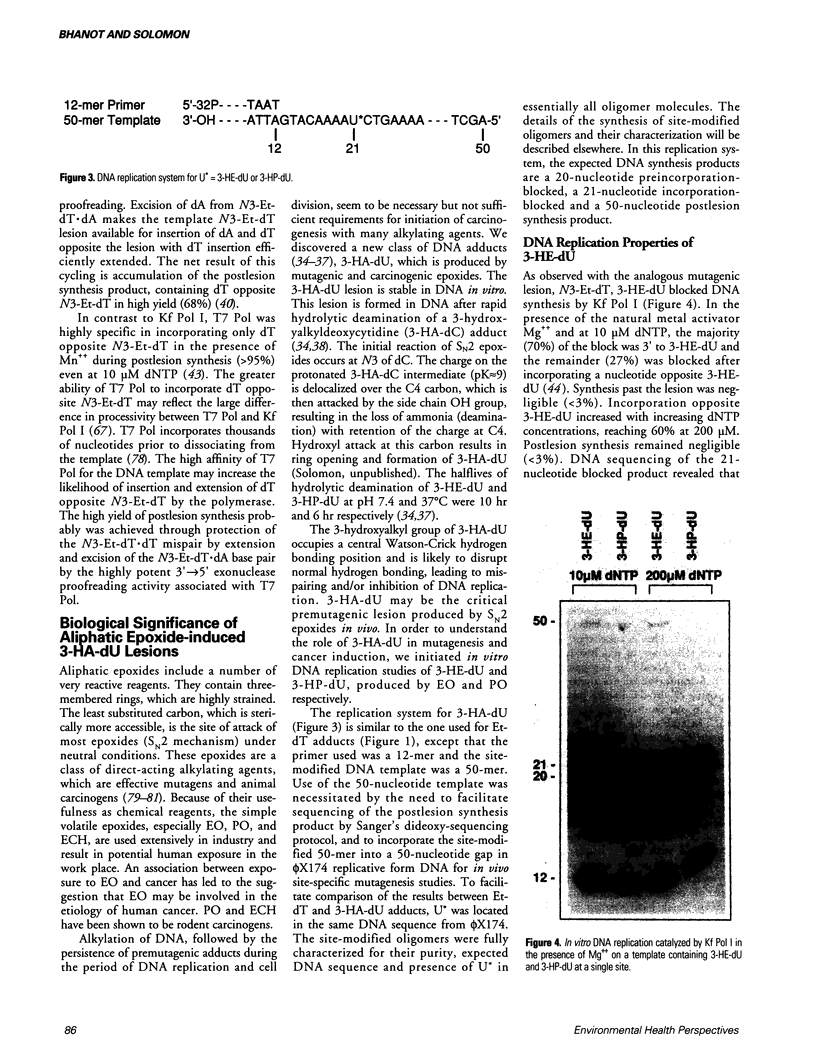
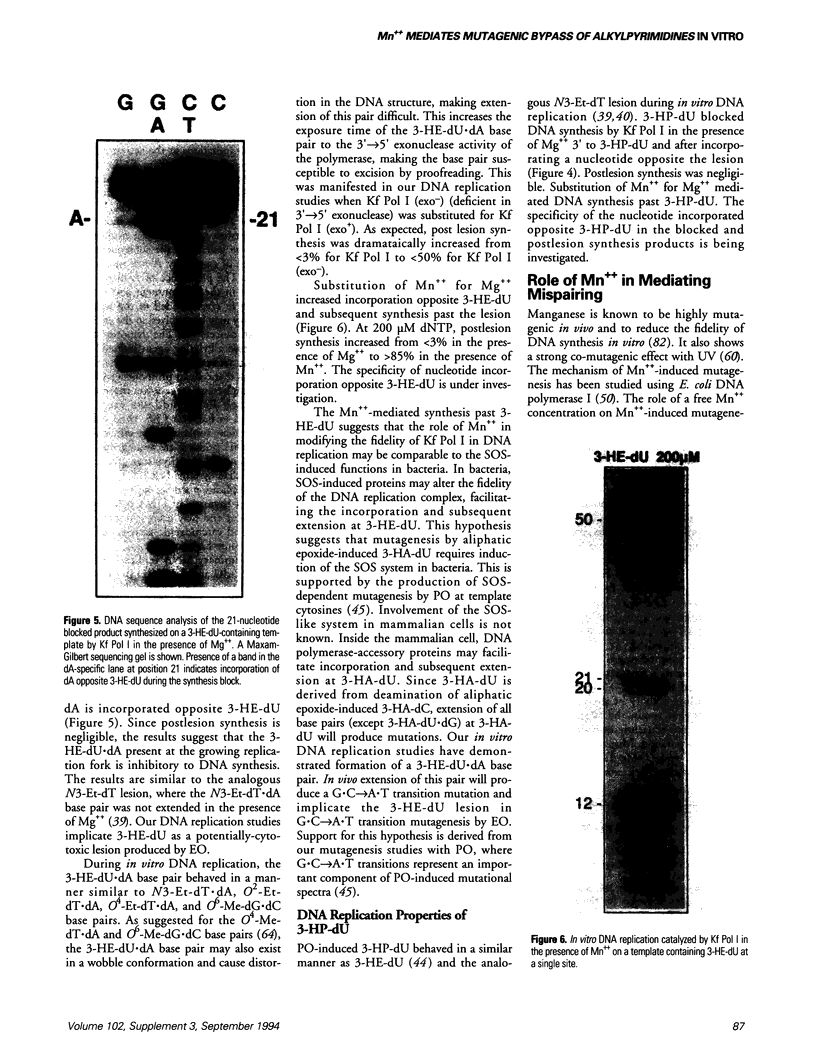
Abstract
A variety of alkylating mutagens and carcinogens produce pyrimidine adducts in DNA that block DNA synthesis in vitro. Since DNA synthesis past the lesion is a necessary step to produce mutations, we investigated the role of the mutagenic metal ion Mn++ in facilitating DNA synthesis past alkylpyrimidines. In the presence of the natural metal activator Mg++, N3-ethyldeoxythymidine (N3-Et-dT) and O2-ethyldeoxythymidine (O2-Et-dT), present at a single site in DNA, blocked in vitro DNA synthesis 3' to the lesion and after incorporating dA opposite each lesion. The presence of Mn++ permitted postlesion synthesis with dT misincorporated opposite N3-Et-dT and O2-Et-dT, implicating these lesions in A.T-->T.A transversion mutagenesis. The DNA synthesis block by O4-ethyldeoxythymidine (O4-Et-dT) in the presence of Mg++ was partial and was also removed by Mn++. Consistent with in vivo studies, dG was incorporated opposite O4-Et-dT during postlesion synthesis, leading to A.T-->G.C transition mutagenesis. We also have discovered a new class of DNA adducts, N3-hydroxyalkyldeoxyuridine (3-HA-dU) lesions, which are produced by mutagenic and carcinogenic aliphatic epoxides. 3-HA-dU is formed after initial alkylation at the N3 position of dC followed by a rapid hydrolytic deamination. As observed with the analogous mutagenic N3-Et-dT, the ethylene oxide-induced 3-hydroxyethyldeoxyuridine (3-HE-dU) blocked in vitro DNA synthesis, which could be by-passed in the presence of Mn++. The nucleotide incorporated opposite 3-HE-dU during postlesion synthesis is being identified. These studies suggest a role for Mn++ in mediating mutagenic and carcinogenic effects of environmentally important ethylating agents and aliphatic epoxides.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Ohshima H., Shuker D. E., Pignatelli B., Calmels S. Exposure of humans to endogenous N-nitroso compounds: implications in cancer etiology. Mutat Res. 1990 May;238(3):255–267. doi: 10.1016/0165-1110(90)90017-6. [DOI] [PubMed] [Google Scholar]
- Basu A. K., Essigmann J. M. Site-specifically modified oligodeoxynucleotides as probes for the structural and biological effects of DNA-damaging agents. Chem Res Toxicol. 1988 Jan-Feb;1(1):1–18. doi: 10.1021/tx00001a001. [DOI] [PubMed] [Google Scholar]
- Beranek D. T. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat Res. 1990 Jul;231(1):11–30. doi: 10.1016/0027-5107(90)90173-2. [DOI] [PubMed] [Google Scholar]
- Bhanot O. S., Grevatt P. C., Donahue J. M., Gabrielides C. N., Solomon J. J. In vitro DNA replication implicates O2-ethyldeoxythymidine in transversion mutagenesis by ethylating agents. Nucleic Acids Res. 1992 Feb 11;20(3):587–594. doi: 10.1093/nar/20.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot O. S., Grevatt P. C., Donahue J. M., Gabrielides C. N., Solomon J. J. Incorporation of dA opposite N3-ethylthymidine terminates in vitro DNA synthesis. Biochemistry. 1990 Nov 13;29(45):10357–10364. doi: 10.1021/bi00497a010. [DOI] [PubMed] [Google Scholar]
- Bhanot O. S., Ray A. The in vivo mutagenic frequency and specificity of O6-methylguanine in phi X174 replicative form DNA. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7348–7352. doi: 10.1073/pnas.83.19.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogovski P., Bogovski S. Animal Species in which N-nitroso compounds induce cancer. Int J Cancer. 1981;27(4):471–474. doi: 10.1002/ijc.2910270408. [DOI] [PubMed] [Google Scholar]
- Boosalis M. S., Petruska J., Goodman M. F. DNA polymerase insertion fidelity. Gel assay for site-specific kinetics. J Biol Chem. 1987 Oct 25;262(30):14689–14696. [PubMed] [Google Scholar]
- Brent T. P., Dolan M. E., Fraenkel-Conrat H., Hall J., Karran P., Laval L., Margison G. P., Montesano R., Pegg A. E., Potter P. M. Repair of O-alkylpyrimidines in mammalian cells: a present consensus. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1759–1762. doi: 10.1073/pnas.85.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein S. M., Cochrane J. E., Craft T. R., Swenberg J. A., Skopek T. R. Toxicity, mutagenicity, and mutational spectra of N-ethyl-N-nitrosourea in human cell lines with different DNA repair phenotypes. Cancer Res. 1991 Oct 1;51(19):5188–5197. [PubMed] [Google Scholar]
- Canter D. A., Zeiger E., Haworth S., Lawlor T., Mortelmans K., Speck W. Comparative mutagenicity of aliphatic epoxides in Salmonella. Mutat Res. 1986 Nov;172(2):105–138. doi: 10.1016/0165-1218(86)90069-8. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. A comparison of associated enzyme activities in various deoxyribonucleic acid polymerases. J Biol Chem. 1973 May 25;248(10):3398–3404. [PubMed] [Google Scholar]
- Den Engelse L., De Graaf A., De Brij R. J., Menkveld G. J. O2- and O4-ethylthymine and the ethylphosphotriester dTp(Et)dT are highly persistent DNA modifications in slowly dividing tissues of the ethylnitrosourea-treated rat. Carcinogenesis. 1987 Jun;8(6):751–757. doi: 10.1093/carcin/8.6.751. [DOI] [PubMed] [Google Scholar]
- Dosanjh M. K., Essigmann J. M., Goodman M. F., Singer B. Comparative efficiency of forming m4T.G versus m4T.A base pairs at a unique site by use of Escherichia coli DNA polymerase I (Klenow fragment) and Drosophila melanogaster polymerase alpha-primase complex. Biochemistry. 1990 May 15;29(19):4698–4703. doi: 10.1021/bi00471a026. [DOI] [PubMed] [Google Scholar]
- Dosanjh M. K., Galeros G., Goodman M. F., Singer B. Kinetics of extension of O6-methylguanine paired with cytosine or thymine in defined oligonucleotide sequences. Biochemistry. 1991 Dec 10;30(49):11595–11599. doi: 10.1021/bi00113a015. [DOI] [PubMed] [Google Scholar]
- Dube D. K., Loeb L. A. Manganese as a mutagenic agent during in vitro DNA synthesis. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1041–1046. doi: 10.1016/0006-291x(75)90779-2. [DOI] [PubMed] [Google Scholar]
- Dunkelberg H. Carcinogenicity of ethylene oxide and 1,2-propylene oxide upon intragastric administration to rats. Br J Cancer. 1982 Dec;46(6):924–933. doi: 10.1038/bjc.1982.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H., Goodman M. F. Fidelity mechanisms in DNA replication. Annu Rev Biochem. 1991;60:477–511. doi: 10.1146/annurev.bi.60.070191.002401. [DOI] [PubMed] [Google Scholar]
- Eckert K. A., Ingle C. A., Drinkwater N. R. N-ethyl-N-nitrosourea induces A:T to C:G transversion mutations as well as transition mutations in SOS-induced Escherichia coli. Carcinogenesis. 1989 Dec;10(12):2261–2267. doi: 10.1093/carcin/10.12.2261. [DOI] [PubMed] [Google Scholar]
- Eckert K. A., Ingle C. A., Klinedinst D. K., Drinkwater N. R. Molecular analysis of mutations induced in human cells by N-ethyl-N-nitrosourea. Mol Carcinog. 1988;1(1):50–56. doi: 10.1002/mc.2940010111. [DOI] [PubMed] [Google Scholar]
- Eichhorn G. L., Shin Y. A. Interaction of metal ions with polynucleotides and related compounds. XII. The relative effect of various metal ions on DNA helicity. J Am Chem Soc. 1968 Dec 18;90(26):7323–7328. doi: 10.1021/ja01028a024. [DOI] [PubMed] [Google Scholar]
- El-Deiry W. S., Downey K. M., So A. G. Molecular mechanisms of manganese mutagenesis. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7378–7382. doi: 10.1073/pnas.81.23.7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R., Shi J. P., Tsui W. C. Kinetics of base misinsertion by DNA polymerase I of Escherichia coli. J Mol Biol. 1983 Apr 25;165(4):655–667. doi: 10.1016/s0022-2836(83)80272-1. [DOI] [PubMed] [Google Scholar]
- Goodman M. F., Keener S., Guidotti S., Branscomb E. W. On the enzymatic basis for mutagenesis by manganese. J Biol Chem. 1983 Mar 25;258(6):3469–3475. [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevatt P. C., Donahue J. M., Bhanot O. S. The role of N3-ethyldeoxythymidine in mutagenesis and cytotoxicity by ethylating agents. J Biol Chem. 1991 Jan 15;266(2):1269–1275. [PubMed] [Google Scholar]
- Grevatt P. C., Solomon J. J., Bhanot O. S. In vitro mispairing specificity of O2-ethylthymidine. Biochemistry. 1992 May 5;31(17):4181–4188. doi: 10.1021/bi00132a005. [DOI] [PubMed] [Google Scholar]
- Guttenplan J. B. Mutagenesis by N-nitroso compounds: relationships to DNA adducts, DNA repair, and mutational efficiencies. Mutat Res. 1990 Nov-Dec;233(1-2):177–187. doi: 10.1016/0027-5107(90)90161-v. [DOI] [PubMed] [Google Scholar]
- Hall Z. W., Lehman I. R. An in vitro transversion by a mutationally altered T4-induced DNA polymerase. J Mol Biol. 1968 Sep 28;36(3):321–333. doi: 10.1016/0022-2836(68)90158-7. [DOI] [PubMed] [Google Scholar]
- Horsfall M. J., Gordon A. J., Burns P. A., Zielenska M., van der Vliet G. M., Glickman B. W. Mutational specificity of alkylating agents and the influence of DNA repair. Environ Mol Mutagen. 1990;15(2):107–122. doi: 10.1002/em.2850150208. [DOI] [PubMed] [Google Scholar]
- Huff A. C., Topal M. D. DNA damage at thymine N-3 abolishes base-pairing capacity during DNA synthesis. J Biol Chem. 1987 Sep 15;262(26):12843–12850. [PubMed] [Google Scholar]
- Huh N. H., Rajewsky M. F. Enzymatic elimination of O6-ethylguanine from the DNA of ethylnitrosourea-exposed normal and malignant rat brain cells grown under cell culture versus in vivo conditions. Int J Cancer. 1988 May 15;41(5):762–766. doi: 10.1002/ijc.2910410521. [DOI] [PubMed] [Google Scholar]
- Lai M. D., Beattie K. L. Influence of divalent metal activator on the specificity of misincorporation during DNA synthesis catalyzed by DNA polymerase I of Escherichia coli. Mutat Res. 1988 Mar;198(1):27–36. doi: 10.1016/0027-5107(88)90036-x. [DOI] [PubMed] [Google Scholar]
- Larson K. L., Strauss B. S. Influence of template strandedness on in vitro replication of mutagen-damaged DNA. Biochemistry. 1987 May 5;26(9):2471–2479. doi: 10.1021/bi00383a011. [DOI] [PubMed] [Google Scholar]
- Lewis J. G., Swenberg J. A. Differential repair of O(6)-methylguanine in DNA of rat hepatocytes and nonparenchymal cells. Nature. 1980 Nov 13;288(5787):185–141. doi: 10.1038/288185a0. [DOI] [PubMed] [Google Scholar]
- Lewis S. E., Johnson F. M., Skow L. C., Popp D., Barnett L. B., Popp R. A. A mutation in the beta-globin gene detected in the progeny of a female mouse treated with ethylnitrosourea. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5829–5831. doi: 10.1073/pnas.82.17.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. F., Reese C. B., Swann P. F. Synthesis and characterization of oligodeoxynucleotides containing 4-O-methylthymine. Biochemistry. 1987 Feb 24;26(4):1086–1093. doi: 10.1021/bi00378a015. [DOI] [PubMed] [Google Scholar]
- Li F., Segal A., Solomon J. J. In vitro reaction of ethylene oxide with DNA and characterization of DNA adducts. Chem Biol Interact. 1992 Jun 15;83(1):35–54. doi: 10.1016/0009-2797(92)90090-8. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Sedgwick B., Sekiguchi M., Nakabeppu Y. Regulation and expression of the adaptive response to alkylating agents. Annu Rev Biochem. 1988;57:133–157. doi: 10.1146/annurev.bi.57.070188.001025. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Dube D. K., Beckman R. A., Koplitz M., Gopinathan K. P. On the fidelity of DNA replication. Nucleoside monophosphate generation during polymerization. J Biol Chem. 1981 Apr 25;256(8):3978–3987. [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. N. The experimental basis for the role of nitroso compounds in human cancer. Cancer Surv. 1989;8(2):207–239. [PubMed] [Google Scholar]
- Maher V. M., Domoradzki J., Bhattacharyya N. P., Tsujimura T., Corner R. C., McCormick J. J. Alkylation damage, DNA repair and mutagenesis in human cells. Mutat Res. 1990 Nov-Dec;233(1-2):235–245. doi: 10.1016/0027-5107(90)90166-2. [DOI] [PubMed] [Google Scholar]
- Mendelman L. V., Boosalis M. S., Petruska J., Goodman M. F. Nearest neighbor influences on DNA polymerase insertion fidelity. J Biol Chem. 1989 Aug 25;264(24):14415–14423. [PubMed] [Google Scholar]
- Mendelman L. V., Petruska J., Goodman M. F. Base mispair extension kinetics. Comparison of DNA polymerase alpha and reverse transcriptase. J Biol Chem. 1990 Feb 5;265(4):2338–2346. [PubMed] [Google Scholar]
- Michaels M. L., Johnson D. L., Reid T. M., King C. M., Romano L. J. Evidence for in vitro translesion DNA synthesis past a site-specific aminofluorene adduct. J Biol Chem. 1987 Oct 25;262(30):14648–14654. [PubMed] [Google Scholar]
- Perantoni A. O., Rice J. M., Reed C. D., Watatani M., Wenk M. L. Activated neu oncogene sequences in primary tumors of the peripheral nervous system induced in rats by transplacental exposure to ethylnitrosourea. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6317–6321. doi: 10.1073/pnas.84.17.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp R. A., Bailiff E. G., Skow L. C., Johnson F. M., Lewis S. E. Analysis of a mouse alpha-globin gene mutation induced by ethylnitrosourea. Genetics. 1983 Sep;105(1):157–167. doi: 10.1093/genetics/105.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston B. D., Singer B., Loeb L. A. Mutagenic potential of O4-methylthymine in vivo determined by an enzymatic approach to site-specific mutagenesis. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8501–8505. doi: 10.1073/pnas.83.22.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin S. D., Strauss B. S. A role for DNA polymerase in the specificity of nucleotide incorporation opposite N-acetyl-2-aminofluorene adducts. J Mol Biol. 1984 Sep 25;178(3):569–594. doi: 10.1016/0022-2836(84)90239-0. [DOI] [PubMed] [Google Scholar]
- Reyland M. E., Lehman I. R., Loeb L. A. Specificity of proofreading by the 3'----5' exonuclease of the DNA polymerase-primase of Drosophila melanogaster. J Biol Chem. 1988 May 15;263(14):6518–6524. [PubMed] [Google Scholar]
- Richardson K. K., Richardson F. C., Crosby R. M., Swenberg J. A., Skopek T. R. DNA base changes and alkylation following in vivo exposure of Escherichia coli to N-methyl-N-nitrosourea or N-ethyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1987 Jan;84(2):344–348. doi: 10.1073/pnas.84.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman T. G., Molina M. The genetic toxicology of metal compounds: II. Enhancement of ultraviolet light-induced mutagenesis in Escherichia coli WP2. Environ Mutagen. 1986;8(2):263–271. doi: 10.1002/em.2860080208. [DOI] [PubMed] [Google Scholar]
- Saffhill R., Margison G. P., O'Connor P. J. Mechanisms of carcinogenesis induced by alkylating agents. Biochim Biophys Acta. 1985 Dec 17;823(2):111–145. doi: 10.1016/0304-419x(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Segal A., Solomon J. J., Mukai F. In vitro reactions of glycidol with pyrimidine bases in calf thymus DNA. Cancer Biochem Biophys. 1990 Jan;11(1):59–67. [PubMed] [Google Scholar]
- Singer B., Chavez F., Spengler S. J., Kuśmierek J. T., Mendelman L., Goodman M. F. Comparison of polymerase insertion and extension kinetics of a series of O2-alkyldeoxythymidine triphosphates and O4-methyldeoxythymidine triphosphate. Biochemistry. 1989 Feb 21;28(4):1478–1483. doi: 10.1021/bi00430a008. [DOI] [PubMed] [Google Scholar]
- Singer B. O-alkyl pyrimidines in mutagenesis and carcinogenesis: occurrence and significance. Cancer Res. 1986 Oct;46(10):4879–4885. [PubMed] [Google Scholar]
- Singer B., Spengler S. J., Fraenkel-Conrat H., Kuśmierek J. T. O4-Methyl, -ethyl, or -isopropyl substituents on thymidine in poly(dA-dT) all lead to transitions upon replication. Proc Natl Acad Sci U S A. 1986 Jan;83(1):28–32. doi: 10.1073/pnas.83.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Infidelity of DNA synthesis in vitro: screening for potential metal mutagens or carcinogens. Science. 1976 Dec 24;194(4272):1434–1436. doi: 10.1126/science.1006310. [DOI] [PubMed] [Google Scholar]
- Skopek T. R., Walker V. E., Cochrane J. E., Craft T. R., Cariello N. F. Mutational spectrum at the Hprt locus in splenic T cells of B6C3F1 mice exposed to N-ethyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7866–7870. doi: 10.1073/pnas.89.17.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan D. L., Loeb L. A., Mildvan A. S. Conformation of deoxynucleoside triphosphate substrates on DNA polymerase I from Escherichia coli as determined by nuclear magnetic relaxation. J Biol Chem. 1975 Dec 10;250(23):8913–8920. [PubMed] [Google Scholar]
- Snow E. T., Singh J., Koenig K. L., Solomon J. J. Propylene oxide mutagenesis at template cytosine residues. Environ Mol Mutagen. 1994;23(4):274–280. doi: 10.1002/em.2850230403. [DOI] [PubMed] [Google Scholar]
- Solomon J. J., Mukai F., Fedyk J., Segal A. Reactions of propylene oxide with 2'-deoxynucleosides and in vitro with calf thymus DNA. Chem Biol Interact. 1988;67(3-4):275–294. doi: 10.1016/0009-2797(88)90064-6. [DOI] [PubMed] [Google Scholar]
- Solomon J. J., Singh U. S., Segal A. In vitro reactions of 2-cyanoethylene oxide with calf thymus DNA. Chem Biol Interact. 1993 Sep;88(2-3):115–135. doi: 10.1016/0009-2797(93)90087-f. [DOI] [PubMed] [Google Scholar]
- Stowers S. J., Wiseman R. W., Ward J. M., Miller E. C., Miller J. A., Anderson M. W., Eva A. Detection of activated proto-oncogenes in N-nitrosodiethylamine-induced liver tumors: a comparison between B6C3F1 mice and Fischer 344 rats. Carcinogenesis. 1988 Feb;9(2):271–276. doi: 10.1093/carcin/9.2.271. [DOI] [PubMed] [Google Scholar]
- Swann P. F. Why do O6-alkylguanine and O4-alkylthymine miscode? The relationship between the structure of DNA containing O6-alkylguanine and O4-alkylthymine and the mutagenic properties of these bases. Mutat Res. 1990 Nov-Dec;233(1-2):81–94. doi: 10.1016/0027-5107(90)90153-u. [DOI] [PubMed] [Google Scholar]
- Swenberg J. A., Dyroff M. C., Bedell M. A., Popp J. A., Huh N., Kirstein U., Rajewsky M. F. O4-ethyldeoxythymidine, but not O6-ethyldeoxyguanosine, accumulates in hepatocyte DNA of rats exposed continuously to diethylnitrosamine. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1692–1695. doi: 10.1073/pnas.81.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. Effect of manganese ions on the incorporation of dideoxynucleotides by bacteriophage T7 DNA polymerase and Escherichia coli DNA polymerase I. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4076–4080. doi: 10.1073/pnas.86.11.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade D. R., Airy S. C., Sinsheimer J. E. Mutagenicity of aliphatic epoxides. Mutat Res. 1978 Nov;58(2-3):217–223. doi: 10.1016/0165-1218(78)90012-5. [DOI] [PubMed] [Google Scholar]
- White R., Woodward S., Leppert M., O'Connell P., Hoff M., Herbst J., Lalouel J. M., Dean M., Vande Woude G. A closely linked genetic marker for cystic fibrosis. 1985 Nov 28-Dec 4Nature. 318(6044):382–384. doi: 10.1038/318382a0. [DOI] [PubMed] [Google Scholar]
- Zakour R. A., Kunkel T. A., Loeb L. A. Metal-induced infidelity of DNA synthesis. Environ Health Perspect. 1981 Aug;40:197–205. doi: 10.1289/ehp.8140197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielenska M., Beranek D., Guttenplan J. B. Different mutational profiles induced by N-nitroso-N-ethylurea: effects of dose and error-prone DNA repair and correlations with DNA adducts. Environ Mol Mutagen. 1988;11(4):473–485. doi: 10.1002/em.2850110408. [DOI] [PubMed] [Google Scholar]