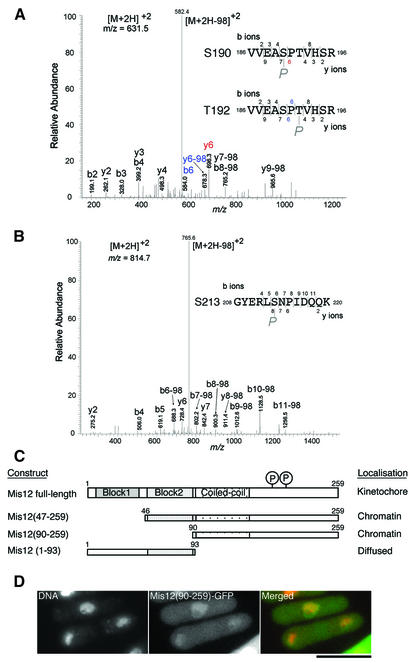
Fig. 7. Residues in the C-terminus of Mis12 are phosphorylated. (A and B) Ser190 or Thr192 and Ser213 are phosphorylated in vivo. HA- and histidine-tagged Mis12 overproduced in wild type was purified using TALON beads under denaturing conditions, and subjected to SDS–PAGE. The band of Mis12-HAHis6 was cut out and trypsinized in-gel, and subjected to survey of the phosphorylation site using MS (Ohta et al., 2002). The MS/MS spectra of the tryptic phosphopeptide amino acids 186–196 (A) and 208–220 (B) of Mis12 obtained by collision-induced dissociation of the [M + 2H]2+precursor ions, m/z 631.5 and 814.7, are shown. In both cases, intense [M + 2H –98]2+ ions, m/z 582.4 in (A) and 765.8 in (B), due to neutral loss (H2O + phosphate, 97.8) of precursor ions were observed, indicating that the potential phosphorylated residues are serine or threonine. Three potential sites of phosphorylation (two serine and one threonine) are found in the peptide 186–196 (A). The fragment ions enable localization of the phosphorylated residue to one of the two central residues, Ser190 and Thr192. An ion fragment at m/z 696.3 is assignable to y6 (red letters) when the peptide is phosphorylated at Ser190, and ion fragments at m/z 584.0 and 678.3 are assignable to b6 and y6-98, respectively, when the peptide is phosphorylated at Thr192 (blue letters). There is one possible residue in the peptide 208–220 (B), and the fragment ions were matched to the peptide phosphorylated at Ser213. (C) Localization of three Mis12-truncated proteins. Each construct is tagged with GFP at the C-terminus and expressed from REP41 plasmid that allows moderate overexpression. (D) Chromatin localization of truncated Mis12 (amino acids 90–259)–GFP protein. DNA was counterstained by DAPI. Bar, 10 µm.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.