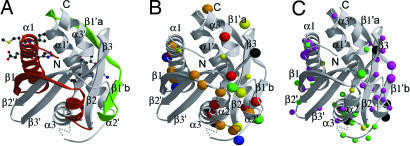
Fig. 4.
Mapping mutations that affect function onto the FliMM structure. (A) Ten-residue deletion mutants in the green region produced paralyzed but flagellated phenotypes (12), which suggests that these regions are not important for flagellar assembly per se but necessary for rotation. Deletions in the red region produced nonflagellated cells and were not dominant when expressed with wild-type FliM. Thus, these proteins could not provide contacts for assembly (12). (B) Solvent-exposed positions of conserved hydrophobic (yellow), negatively charged (red), positively charged (blue), polar (green), and glycine (black) residues in FliMM. Larger sphere size indicates invariant residues. Orange indicates residues that are conserved by proteins within each of two families of chemotactic bacteria but differ in residue type between the two families (Fig. 6). (C) Positions of single-point mutations on exposed residues of FliMM that suppress cheY or cheZ mutants and cause either CW motor bias (magenta spheres) or CCW motor bias (green spheres), respectively. The two classes of mutants occur in distinct regions, divided by an area where mutations can cause either phenotype (yellow spheres) (35). Positions of mutations that result in paralyzed phenotypes are shown as black spheres (35). Larger-size spheres indicate mutations that cause greater shifts in the switch bias.