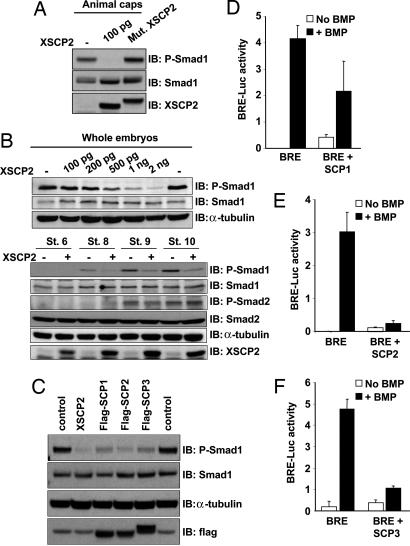
Fig. 2.
XSCP2 and human SCPs cause Smad1 C-terminal dephosphorylation in vivo. (A) Overexpression of XSCP2 in animal caps decreases endogenous Smad1 C-terminal phosphorylation level. Two-cell-stage embryos were injected in the animal pole of both blastomeres with 100 pg of XSCP2 or Mut.XSCP2 mRNAs or left untreated. Animal cap explants were isolated at stage 9 and cultured until stage 10.5. Lysates were prepared and analyzed by immunoblotting with anti-P-Smad1, anti-Smad1, and anti-XSCP2 antibodies. The difference in electrophoretic mobility between Mut.XSCP2 and XSCP2 is due to the presence of a flag tag on the mutant form. (B) (Upper) XSCP2 effect on endogenous Smad1 phosphorylation level is dose dependent. Embryos were injected with increasing doses of XSCP2 mRNA and processed as in A except that the whole embryo was harvested to prepare the lysate. (Lower) XSCP2 overexpression decreases Smad1 phosphorylation levels without affecting Smad2. Two-cell-stage embryos were injected in both blastomeres with XSCP2 mRNA (1 ng) or left untreated and harvested at the indicated stages. Lysates were analyzed as in A by immunoblotting with the indicated antibodies. As a loading control, the anti-P-Smad1 membrane was stripped and reprobed with anti-α-tubulin. (C) Two-cell-stage embryos were injected with XSCP2 (1 ng), flag-SCP1, flag-SCP2, or flag-SCP3 mRNAs (500 pg), and whole embryos were harvested at stage 10.5. Samples were prepared and analyzed as described in B. (D–F) Luciferase assays with BRE and human SCP1 (D), SCP2 (E), and SCP3 (F). Embryos were injected with the indicated mRNAs and analyzed as in Fig. 1D.