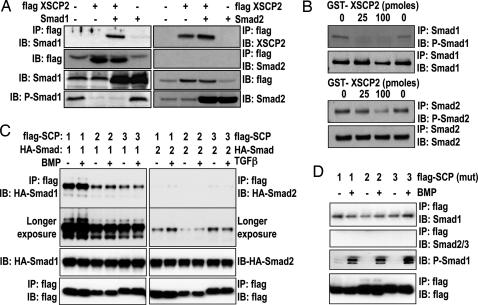
Fig. 3.
SCPs selectively bind and dephosphorylate Smad1. (A) Two-cell-stage embryos were left untreated or injected with the indicated combinations of the following RNAs: Smad1 (2 ng), Smad2 (400 pg), and flag-XSCP2 (2 ng). Embryos were harvested at gastrula stage (stage 10.5). Lysates were prepared and immunoprecipitated with anti-flag M2 antibody and analyzed by immunoblot with anti-Smad1, anti-Smad2, and anti-XSCP2 antibodies. The expression of flag-XSCP2, Smad1, P-Smad1, and Smad2 was checked by immunoblotting the crude extracts used for the IP reaction. (B) In vitro XSCP2 phosphatase assay. Immunoprecipitated Smad1 (Upper) and Smad2 (Lower) from gastrula-stage embryos were incubated with increasing amounts of recombinant GST-XSCP2. Their phosphorylation status was monitored by immunoblotting with corresponding phospho-specific antibodies. To make sure that the same amounts of Smad1 and 2 were immunoprecipitated in the different conditions, we also probed with Smad1 and Smad2 antibodies after the IP. (C) HEK293 cells were transfected with flag-SCP1–3 and HA-Smad1 or HA-Smad2 as indicated, stimulated with BMP or TGF-β for 1 h, and lysed. Flag immunoprecipitates or lysates were immunoblotted with the indicated antibodies. The second blot from the top shows a longer exposure of the uppermost blot to demonstrate weak interaction of SCP1–3 with Smad2. (D) Same as C except that cells were transfected with catalytically inactive mutants of flag-SCP1–3 and treated with or without BMP for 1 h. P-Smad1 was used as a control for BMP stimulation efficiency.