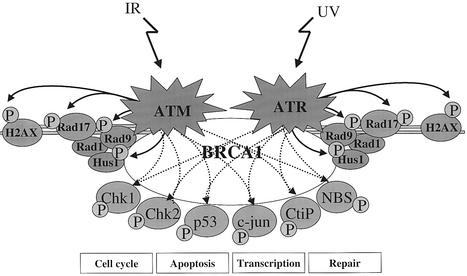
Fig. 6. Model of the role of BRCA1 in damage response signalling. ATM and ATR respond to DNA damage induced by IR or UV, respectively. ATR is associated with ATRIP at the damage sites (not shown for simplicity). Rad17 is recruited to the damage sites independently of ATR, and recruits Rad9, Rad1 and Hus1. BRCA1 is also recruited to the damage sites, in part by an interaction with Rad9 and Hus1 that occurs in undamaged cells. A Rad17–Rad1–Rad9–Hus1 complex also forms at the damage sites after IR treatment but the requirements for its assembly are not well understood. ATR phosphorylates H2AX, Rad17, Rad9 and Hus1 in the absence of BRCA1, possibly because these substrates are directly attached to the DNA. ATM or ATR also phosphorylates a range of additional substrates including Chk1, Chk2, p53, c-Jun, Nbs1 and CtIP, which are required to effect damage-response processes including checkpoint delay and apoptosis. Although the figure shows symmetrical pathways for ATM and ATR phosphorylation events, there are likely to be differences in their requirements. For ATR, the downstream phosphorylation events require BRCA1 and, most likely, the assembly of the Rad17–Rad9–Rad1–Hus1 complex. For ATM, phosphorylation of these substrates also requires the presence of BRCA1, but any requirement for the Rad17 complex is currently unknown. ATM may also require Nbs1 for phosphorylating these substrates (Girard et al., 2002). A requirement of BRCA1 for the phosphorylation of Smc1 and Chk1 by ATM has also recently been demonstrated (Kim et al., 2002; Yarden et al., 2002). We propose that BRCA1 acts as a scaffold to facilitate the downstream phosphorylation of ATM and ATR substrates. BRCA1-dependent phosphorylation events are shown as solid lines, and BRCA1-independent phosphorylation events are represented by broken lines.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.