Abstract
Mass loss from evolved stars results in the formation of unusual chemical laboratories: circumstellar envelopes. Such envelopes are found around carbon- and oxygen-rich asymptotic giant branch stars and red supergiants. As the gaseous material of the envelope flows from the star, the resulting temperature and density gradients create a complex chemical environment involving hot, thermodynamically controlled synthesis, molecule “freeze-out,” shock-initiated reactions, and photochemistry governed by radical mechanisms. In the circumstellar envelope of the carbon-rich star IRC+10216, >50 different chemical compounds have been identified, including such exotic species as C8H, C3S, SiC3, and AlNC. The chemistry here is dominated by molecules containing long carbon chains, silicon, and metals such as magnesium, sodium, and aluminum, which makes it quite distinct from that found in molecular clouds. The molecular composition of the oxygen-rich counterparts is not nearly as well explored, although recent studies of VY Canis Majoris have resulted in the identification of HCO+, SO2, and even NaCl in this object, suggesting chemical complexity here as well. As these envelopes evolve into planetary nebulae with a hot, exposed central star, synthesis of molecular ions becomes important, as indicated by studies of NGC 7027. Numerous species such as HCO+, HCN, and CCH are found in old planetary nebulae such as the Helix. This “survivor” molecular material may be linked to the variety of compounds found recently in diffuse clouds. Organic molecules in dense interstellar clouds may ultimately be traced back to carbon-rich fragments originally formed in circumstellar shells.
Keywords: asymptotic giant branch stars, astrochemistry, molecular line observations
Circumstellar envelopes of evolved stars are among the most remarkable chemical laboratories in the universe. These envelopes are created by extensive mass loss in the later stages of stellar evolution, caused by thermal pulses in the star interior and radiation pressure on dust (1, 2). Because of the low temperature of the central star, and the long time scales for mass loss, molecules and dust form in the envelope, and are then gently blown into the interstellar medium (ISM). The material lost from such envelopes is thought to account for nearly 80% (by mass) of the ISM (3).
The Origin of Circumstellar Envelopes
Chemically rich circumstellar shells are found around asymptotic giant branch (AGB) stars and red supergiants (RSGs). AGB stars are objects of low and intermediate initial mass (≈1–8 M⊙) with effective temperatures near T ≈2,000–3,500 K (4–6). These objects occur on an evolutionary track that begins at the end of the main sequence. At this stage, all hydrogen in the stellar core has been converted to helium, and the core itself starts to gravitationally contract. This contraction ignites hydrogen in a shell around the core, and the star enters the so-called red giant branch. As the core continues to contract, the envelope of the red giant expands and becomes convectively unstable. Given sufficient contraction, the helium core will eventually ignite as the H shell continues to burn. The fusion of helium produces 12C, and alpha capture on 12C yields 16O in the core. Eventually, the central helium is exhausted, leaving an oxygen/carbon core surrounded by He-burning and H-burning shells. The appearance of this two-shell structure marks the beginning of the AGB phase (4). The primary source of luminosity in an AGB star is the H-burning shell, but periodically the He shell ignites for short periods of time (5). The energy released in this so-called “thermal pulse” creates a convective zone extending from shell to shell, which mixes nuclear products from the He-burning zone to the stellar surface. This process, called the “third dredge-up,” brings carbon and other elements into the detaching envelope. The thermal pulses also aid in driving the outer material from the star, creating a circumstellar shell (1, 2). In fact, AGB stars lose up to 80% of their original mass in the form of an envelope, with typical rates of 10−6 to 10−4 M⊙·yr−1 (1, 2, 4). They therefore play a major role in the chemical evolution of the galaxy (7).
RSGs evolve from massive O and B stars (M ≈8–30 M⊙) that have left the main sequence. These objects experience intense mass loss (≈10−5 to 10−3 M⊙·yr−1) for ≈103 to 104 yr before they become supernovae, although there are many uncertainties connected with their evolution (2). They typically have surface temperatures of T ≈ 3,500–4,000 K.
The Chemical Environment of Circumstellar Shells
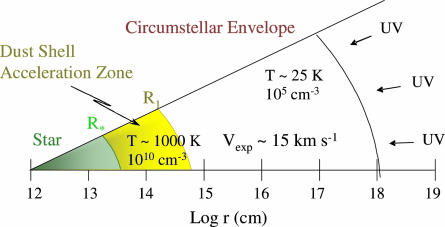
Circumstellar shells exhibit a range of physical conditions, as shown in Fig. 1. In Fig. 1, a simplified cross section of a typical envelope for an AGB star is displayed, plotted on a scale of log r (r = distance from star). These objects are quite large, extending 104 to 105 stellar radii (R*). Near the stellar photosphere, the envelope material (all gaseous) is warm (T ≈1,500 K) and dense (n ≈1010 cm−3). As the matter flows away from the star, it expands and cools, typically with T ∝ r−1 and n ∝ r−2 (8, 9). Dust begins to form at ≈5–15 R*, and shortly thereafter the maximum outflow velocity is achieved. Beyond the dust formation zone, the temperature and density continue to decrease such that at the outer edges of the shell, T ≈25 K and n ≈105 cm−3 (8, 9).
Fig. 1.
Cross section of a circumstellar envelope, plotted in terms of log r, where r is the distance from the central star. Regions of LTE chemistry, dust formation, and UV-induced photochemistry are shown.
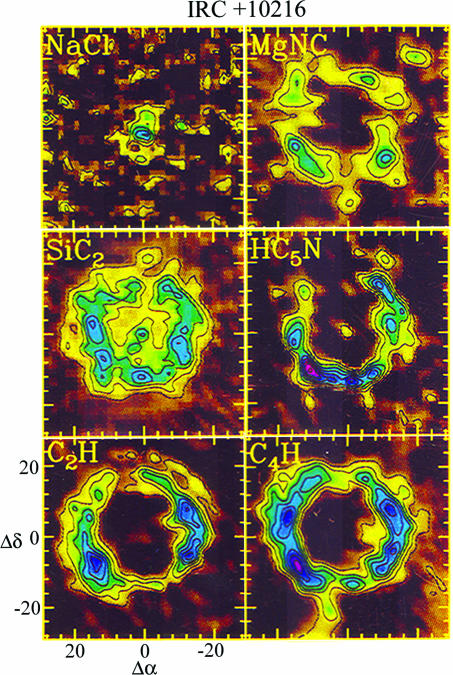
The chemistry varies substantially throughout the envelope. In the inner shell, the temperature and density are sufficiently high that thermodynamic equilibrium governs molecular abundances. Such local thermodynamic equilibrium (LTE) chemistry was predicted in the early models of Tsuji (10) and incorporated into later calculations (e.g., refs. 11 and 12). The LTE predictions have been borne out by observations (13, 14). Infrared spectroscopy has demonstrated that stable, closed-shell species such as CS, NH3, HCN, and HCCH have high abundances in the inner envelope of IRC+10216 (≈10−3 to 10−6, relative to H2). As molecules flow outward, some abundances freeze out at intermediate radii, HCN and CO, for example (15, 16). At the outer regions, photons and cosmic rays initiate an active photochemistry that creates radicals (11, 12). As shown in interferometer maps (17, 18), radicals such as CN, CCH, C3H, and C4H exist in similar shell-like structures near the envelope edge (Fig. 2). They are the daughter species of parent molecules such as HCN and HCCH produced at LTE. In addition, shock waves in the shell, caused by thermal pulses, may influence abundances (19, 20).
Fig. 2.
Maps from the Plateau de Bure interferometer showing the molecular distributions of various species in the envelope of IRC+10216 (17, 18). MgNC, C2H, HC5N, and C4H all exist in the outer shell, whereas NaCl is an inner envelope species. SiC2 is present in the intermediate regions.
There are additional complications to this scheme. For example, some shells are carbon-rich (C type), whereas others are oxygen-rich (M type), and S types have C ≈ O. AGB envelopes are thought to initially start with more oxygen than carbon; they become C-enriched during the third dredge-up (4, 5). Shocks and pulsations associated with the third dredge-up may also facilitate the chemistry, because C-rich envelopes appear to have the greatest variety of chemical compounds. Other complexities come into play with post-AGB evolution and the formation of so-called “protoplanetary nebulae” (PPN). Observations have suggested that during the PPN phase, a second, more violent mass loss event occurs with high velocity winds that partially destroy the remnant AGB envelope (e.g., refs. 21–23). For example, CRL 2688, the Egg Nebula, exhibits both low (<20 km·s−1) and high velocity (>100 km·s−1) outflows (24, 25). A more evolved object is CRL 618; here, the star, which has lost its dusty shroud, is producing sufficient UV radiation to create a small, nearby HII region (26, 27).
The envelopes of RSGs also have large temperature and density gradients, a dust formation zone, and significant molecular abundances, substantiated by various observations (e.g., refs. 3, 13, 28, and 29). However, RSG stars have shorter lifetimes than their AGB counterparts (a few hundred thousand years), and their mass loss appears to be more sporadic and asymmetric, characterized by jets and high-density streams (30, 31). The impact of these differences on the chemistry of RSG shells has yet to be evaluated.
Distinct Chemical Aspects: Carbon, Silicon, and Metals
Circumstellar shells are principally studied by infrared and millimeter/submillimeter astronomy. At millimeter/submillimeter wavelengths, the pure rotational transitions of numerous circumstellar molecules have been measured, providing a wealth of chemical information (e.g., ref. 32). Infrared observations probe the inner regions and have identified species with no permanent dipole moment (CH4, SiH4) and provided data on dust (13, 14, 33, 34). In the envelope of IRC+10216, the best-studied AGB star at a distance of ≈150 parsec (pc = 3.09 × 1016 m), >50 different chemical compounds have been identified, as shown in Table 1. About 80% have been detected by radio/mm astronomy. In addition, there are many unidentified features in the mm spectrum that arise from unknown species (e.g., ref. 35).
Table 1.
Confirmed molecular identifications in IRC+10216
| CO | CCH | HC3N | CCS | SiO | NaCl |
| CS | C3H | HC5N | C3S | SiS | AlCl |
| CN | C3O | HC7N | C3N | SiC | KCl |
| HCN | C4H | HC9N | C5N | SiN | AlF |
| HCCH | C5H | H2C4 | HC4N | SiC2 | MgNC |
| HNC | C6H | H2C6 | c-C3H2 | SiC3 | MgCN |
| H2CCH2 | C7H | HC2N | CH3CN | SiCN | AlNC |
| CH4 | C8H | C3 | CP | SiC4 | KCN |
| NH3 | C2 | C5 | PN | SiH4 | NaCN |
| H2S |
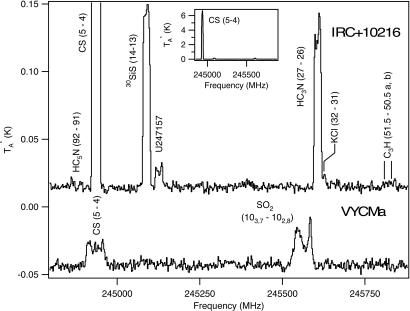
A representative spectrum from IRC+10216, observed at 245 GHz, is shown in Fig. 3 Upper. The 3σ noise level in these data are <10 mK (TA*). This spectrum illustrates the striking chemistry of C-rich AGB shells. First of all, the spectral lines present arise primarily from molecules containing carbon. About 80% of all species identified in this object contain this element, and there are several series of acetylenic chains, including HCnN (n = 1, 3, 5, 7, 9) and CnH (n = 2, 3, 4, 5, 6, 7, 8), and unusual radicals such as H2C4, H2C6, and HC4N. Chemical modeling can reproduce the abundances of many of the chains by using ion–molecule reactions (12). It is unclear, however, whether such reactions are relevant because there has never been a conclusive detection of any ion in IRC+10216 to my knowledge. Radical–radical reactions have rates approaching those of ion–molecule processes, and these may be responsible for the interesting array of compounds.
Fig. 3.
Observations of spectral lines toward the carbon-rich shell of IRC+10216 (Upper and Inset) and the oxygen-rich envelope of VYCMa (Lower) at 245 GHz, measured with the SMT telescope of the Arizona Radio Observatory with an Atacama Large Millimeter Array Band 6 mixer.
Also present in the IRC+10216 spectrum is a line arising from 30SiS. AGB envelopes of carbon-rich stars are the only sources that contain a wide assortment of silicon species, nine in all (see Table 1). Molecular clouds typically contain SiO and sometimes SiS (36, 37). Other simple silicon-bearing species such as SiC, SiN, and SiC2 have yet to be identified in such objects.
In addition, AGB shells are the only sources where metal-containing molecules have been securely identified. As shown in Table 1, nine such compounds have been observed in IRC+10216. The species fall into two basic categories: (i) metal halides (NaCl, KCl, AlCl, and AlF) and (ii) metal cyanide/isocyanide species (MgNC, MgCN, NaCN, AlNC, and KCN). Although the presence of metal halides such as NaCl (salt) seems unusual, these species were predicted by the early models of Tsuji (10). These calculations showed that metal halides form in the inner shell under LTE conditions, but disappear from the gas phase at the onset of dust formation. Line profiles and high spatial resolution maps indicate that the halides are confined to the inner shell, as shown in Fig. 2 (17, 18, 38, 39). The metal cyanides and isocyanides, on the other hand, were not predicted by any model, and their discovery was unanticipated. Moreover, MgNC and its metastable isomer, MgCN, are both radicals and exist in the outer envelope, as indicated by their U-shaped line profiles (40–42). In fact, interferometer maps (Fig. 2) have shown that MgNC is located in a narrow shell coincident with the distributions of C2H, HC5N, and C4H (42). AlNC and KCN also exhibit U-shaped profiles, showing that they have extended, outer envelope components (35). In contrast, NaCN is located in the inner shell (18).
The metal cyanides/isocyanides are unusual chemical compounds in that their geometries vary from linear to T-shaped structures; they are also the major carriers of metals in circumstellar gas. Furthermore, four of these species are present in the cold and dense gas of the outer shell and well beyond the dust formation zone. The appearance of these species at the shell edges is salient evidence that not all refractory material condenses into dust grains.
The formation of metal cyanide/isocyanide species in the outer shell has not been satisfactorily explained. The only theory thus far suggests that these compounds are created by the radiative association of a positive metal ion M+ and a carbon chain such as HC5N (43):
The reaction complex MNC5H+ is stabilized through vibrational relaxation. Dissociative recombination of this ion with an electron produces MNC, but the branching ratios are not known:
It is interesting to note that MgNC and C4H have nearly identical distributions in IRC+10216 (42), suggesting that this scheme may be correct (Fig. 2).
Na, Mg, Al, and K are not the four most abundant metals. Magnesium is as common as silicon, cosmically (Mg/H ≈Si/H ≈3 × 10−5), but the next most abundant metal is iron (Fe/H ≈8 × 10−6). An Fe-containing molecule has yet to be observed in circumstellar gas. Aluminum, sodium, calcium, and nickel have similar abundances (≈2 × 10−6), but Ca- and Ni-bearing molecules have not been identified. Potassium is an order of magnitude less abundant than Al, Ca, Na, and Ni. Clearly cosmic abundances alone are not responsible for the observed species. AGB stars, on the other hand, are thought to be significant producers of certain metals, including Na, Mg, and perhaps Al (4, 5). The metal compounds observed may reflect enhancements by AGB nucleosynthesis and subsequent dredge-up.
These conclusions have been based on results for one object, IRC+10216. As discussed by Olofsson (44), few detailed studies of other AGB envelopes exist. CO, HCN, HC3N, CN, and CS have been routinely observed in other carbon-rich envelopes such as CIT 6, AFGL 3068, and IRC+40540, as have SiO and SiC2 (e.g., refs. 45 and 46). More attention has been given to C-rich PPN, in particular CRL 2688 and CRL 618 (e.g., refs. 23, 26, 27, and 47). In CRL 618, the chemistry is dramatically altered by UV radiation, indicated by appearance of ions (HCO+) and an enhancement of the cyanopolyyne chains (48). These PPN are the only other sources of metal-containing species, with the exception of one O-rich star (see below). NaCl, MgNC, and NaCN have been identified in CRL 2688, and MgNC has been identified in CRL 618 (23, 47).
Chemical Variations in Oxygen-Rich Envelopes
Oxygen-rich circumstellar shells have not been studied as yet with the detail of IRC+10216. There have been limited observations of SiO, HCN, SO, CS, and HC3N (46). TX Cam, a Mira variable with a mass loss rate of 3 × 10−6 M⊙·yr−1, appears to be one of the more chemically active O-rich envelopes. Here HCN, CN, CO, CS, SiS, SiO, and SO have been detected (46, 49). The other interesting O-rich source is OH 231.8, a PPN with a bipolar outflow, located ≈1.5 kpc away. In this object, CO, HCO+, SO, SO2, H2S, NS, HCN, CS, and HNC have been identified, along with maser emission from OH, H2O, and SiO (50, 51). However, such objects appear synthetically poor relative to IRC+10216 and suggest that the chemistry of O-rich envelopes is not nearly as complex as their carbon analogs. Furthermore, they do not appear to contain many unidentified lines.
To examine the chemical complexity of oxygen-rich envelopes, a sensitive mm spectral line survey is being conducted of the supergiant VY Canis Majoris (VYCMa) by using the Arizona Radio Observatory. The O-rich shell of this RSG star is thought to have a mass loss rate comparable to IRC+10216 (≈2–3 × 10−4 M⊙·yr−1) and is located at a distance of 1.5 kpc. Preliminary observations suggest that VYCMa has a more complex chemistry than previously thought. As summarized in Table 2, almost as many chemical compounds have been identified in this source as OH 231.8, including SO, SO2, CS, and HCO+, and even NaCl, a third object with salt (S. Milam, A. J. Apponi, N. Woolf, and L.M.Z., unpublished data). In addition, numerous unidentified lines have been found, features not seen in IRC+10216. The observation of HCO+, based on four separate rotational transitions, is solid evidence that a molecular ion exists in a circumstellar envelope, as opposed to a PPN (OH 231.8, CRL 618).
Table 2.
Molecules detected in VYCMa
| H2O | CS | NaCl |
| OH | SO | HCO+ |
| SiO | SO2 | HCN |
| SiS | CO | NH3 |
The contrast between VYCMa and IRC+10216 is shown in Fig. 3, which displays data in the same frequency range from the O-rich star (Fig 3 Lower). Although CS is visible in VYCMa, it is clearly more intense in IRC+10216. Also, the carbon chains are missing in the data of VYCMa, but a line of SO2 is present, with an unusual profile. SO2 is not visible in the IRC+10216 spectrum.
Circumstellar Dust
The dust composition in circumstellar shells is thought to reflect that of the gas phase (34). In the majority of O-rich envelopes (≈4,000), amorphous silicates have been identified on the basis of 9.7- and 18-μm solid-state features. Evidence for crystalline silicates such as olivine and pyroxene has also been found. Other constituents may be refractory oxides of aluminum, silicon, magnesium, and iron. In C-rich stars, the dust is thought to be primarily SiC, which has a 11.3-μm feature arising from the Si-C stretch. It has been observed in >500 AGB stars, but becomes weaker in more evolved objects. In PPN and planetary nebulae (PN), strong infrared features at 3.3, 6,2, 7.7, 8.6, and 11.3 μm are present, which can be attributed to aromatic C-H and C-C stretches and bends, the so-called aromatic infrared bands. There are also bands at 3.4 and 6.9 μm attributed to aliphatic C-H modes. All of these features likely arise from some large solid-state carbon-bearing compound consisting of hundreds of atoms (34).
Into the PN Phase
The mass loss from PPN is particularly energetic, and over a period of ≈1,000 years, the envelope is depleted, gradually exposing the central star (34). As the envelope thins, the stellar radius decreases, in turn raising the temperature of the star to T >30,000 K. The UV output from the star begins to ionize the remnant envelope, forming a PN.
PN are unusually bright and colorful objects because of their atomic emission-line spectra. Remarkable morphologies appear as these nebulae form, including bipolar jets, clover-leaf structures, disks, rings, and spirals (52, 53). Given the presence of the high UV field, and the predicted time scale for photo-dissociation (≈100 years), it was initially thought that all molecular gas in these sources would be destroyed. It has become increasingly apparent over the last 10 years, however, that molecules survive in significant quantities in PN (e.g., refs. 54 and 55); this result was in fact predicted by Hartquist and collaborators (56–58) long before any observations. The persistence of molecular gas is thought to originate from Parker instabilities in the AGB and PPN stages, which create large fragments in the shells that remain into the PN phase (58). It is speculated that the clumps, which are dusty, dense (≈105 to 106 cm−2), and cold (T ≈10–25 K), are in pressure equilibrium with the ionized gas. Molecules in these knots are consequently shielded from photo-destruction and survive despite UV radiation (58).
A list of molecules found in PN is presented in Table 3. Again, the variety of species is limited relative to IRC+10216. Table 3 also reflects abundances of one PN, NGC 7027. This source may not be typical, as it is young (<1,000 yr) and has an extremely hot central star (T* ≈200,000 K). Based on NGC 7027 (59), these sources appear to have a chemistry centered around radicals (OH, CH, CN, and CCH) and ions (HCO+, CH+, N2H+, and CO+), reflecting the copious amounts of UV radiation in these regions. The refractory chemistry of silicon and metals, which is still active in PPN such as CRL 2688, is not apparent. Furthermore, calculations have suggested that the chemistry is quite dynamic, and molecules from the remnant envelope have probably been photo-dissociated and reformed more than once (60).
Table 3.
Molecules identified in PN
| CO | HCN | HCO+ |
| CN | HNC | CO+ |
| CH | CCH | N2H+ |
| OH | H2 | CH+ |
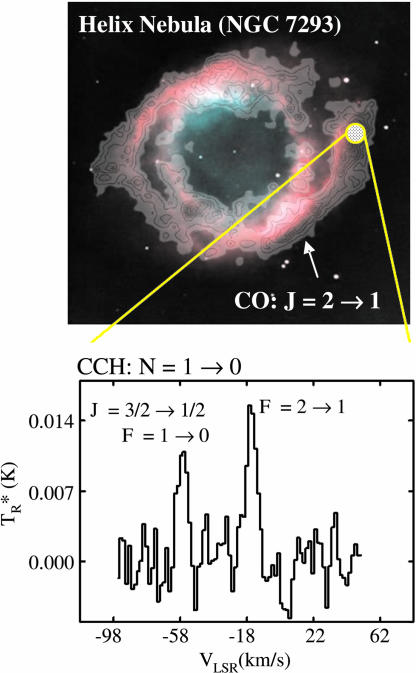
Only limited chemical studies of other PN have been conducted. CO, CN, HCN, HNC, and HCO+ are common to these objects, including highly evolved ones such as NGC 7293, the Helix Nebula (54), which is quite old (≈104 yr). Recent observations have also found that CCH exists in evolved PN, as predicted by Howe, Hartquist, and Williams (58). As shown in Fig. 4, CCH has been identified by two hyperfine components of the N = 1 → 0 transition near 87 GHz in the Helix, as well as in the Ring Nebula, M 4-9, and NGC 6781 (61). The spectrum was measured at the position of peak CO emission (53), as indicated by the overlaid contours on the optical image. CCH and CO appear to be present in gas just outside the ionized nebula, illustrating two important points: (i) the molecular gas is probably critical in determining PN morphology, and (ii) carbon-rich chemistry of the earlier AGB star may be partly preserved into the PN stage.
Fig. 4.
Map of the CO (J = 2 → 1) emission (53) superimposed over the optical image of the PN NGC 7293 (Helix Nebula), as well as a spectrum of CCH observed at the strongest CO position, measured with the Arizona Radio Observatory 12-m telescope. The two hyperfine components of the N = 1 → 0, J = 3/2 → 1/2 transition are present, indicated by the F quantum number.
Molecular Recycling Into Diffuse Clouds?
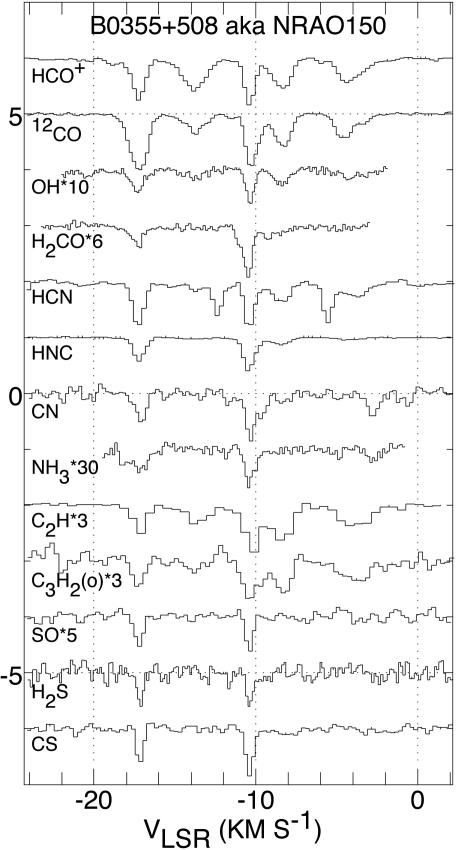
What becomes of the molecular gas in old, dispersing, PN? Some insight may be gained by looking at observational data from diffuse clouds obtained by Liszt et al. (62) and Lucas and Liszt (63). Those authors have detected numerous molecules in these clouds, which have average densities of ≈1–10 cm−3. They discovered these “hidden” species by observing absorption spectra against background quasars. A sample of their detections toward one object, NRAO 150, is displayed in Fig. 5. The molecules observed include HCO+, HCN, OH, HNC, CN, CO, and CCH, a list almost identical to what has routinely been detected in PN. Also, diatomic species commonly found in diffuse gas such as CH, OH, and CH+ (64) are present in such nebulae. [There are additional molecules in diffuse gas (C3H2, NH3, H2S, and H2CO), which perhaps in due course may be found in PN.]
Fig. 5.
Molecular spectra obtained from diffuse clouds along the line of sight toward NRAO 150 (62, 63). The intensity scale is f e−τ, where f = 1 unless otherwise indicated and τ is the optical depth of the molecular transition.
The abundances of the more complex molecules in diffuse clouds are f ≈0.1–10 × 10−8, relative to H2 (62), at least several orders of magnitude higher than predicted by models (e.g., ref. 64), and almost comparable to those found in the dense dark cloud TMC-1. Because molecules are readily destroyed by photo-dissociation in the low densities of diffuse gas, with lifetimes of ≈10–100 yr (64), these detections pose a difficult puzzle for interstellar chemistry. On the other hand, some fraction of the self-shielding knots from PN may continue to persist as the shell material disperses into the interstellar medium. The molecular material in diffuse clouds could be connected to these self-preserving globules.
Eventually diffuse gas gravitationally collapses into more dense objects, creating so-called molecular clouds. Some molecular content of the diffuse gas may survive during this process. If so, diffuse clouds could be seeding dense cloud chemistry, which, at some level, may be traceable back to circumstellar shells and PN.
Such molecular cycling might solve two current puzzles in dense cloud synthesis. First, it would mean that the chemistry in such objects begins with molecules as large as five atoms. It is therefore likely that more complex species can be created within a typical cloud lifetime (≈106 yr). The formation of complex compounds such as methyl formate has been a dilemma for ion–molecule models for some time (e.g., ref. 65). It is interesting to note that the source with the most complex species is SgrB2(N), a cloud near the galactic center. As shown in Fig. 6, which is published as supporting information on the PNAS web site, this object contains a wide variety of organic molecules (66). The galactic center has undergone many cycles of star formation, including processing by AGB stars, as demonstrated by the high 13C/12C ratios found (67).
A second dilemma is the large fraction of species containing carbon-carbon bonds in dense clouds. As Fig. 6 illustrates, many molecules present in such objects contain more than one carbon atom, including CH3CHO, CH3CH2OH, and CH3CH2CN. Yet, in the general interstellar medium, O > C by a factor of ≈1.6 (68), and CO is the second most abundant interstellar molecule. Given the relative abundances of these two elements, most of the carbon should be in the form of CO, leaving little left for organic compounds. However, an active organic chemistry could be initiated in dense clouds if they were seeded by C-C bonds, which remained sufficiently intact through the course of stellar/interstellar evolution after circumstellar formation.
Organic compounds are also found in meteorites such as Murchison and Tagish Lake (69). The high D/H ratios found in some of these compounds are decisive evidence that they originated in cold, interstellar clouds. There is some thought that the bulk of carbon on Earth today was brought from interstellar space via meteorites, comets, and interplanetary dust particles (70, 71). This organic material may have led to living systems, but its ultimate origin could be coupled to the carbon-rich chemistry of evolved stellar envelopes.
Conclusions and Remaining Questions
The chemistry occurring in evolved stars has many distinctive features, in particular carbon, silicon, and metal-enhanced molecular synthesis. Aspects of this chemistry appear to be preserved as these objects progress into PN. The amazing similarity between compounds found in diffuse clouds and PN suggests a connection. The complex chemistry of dense clouds could be better explained if diffuse gas provided the molecular starting materials, in particular carbon–carbon bonds.
Within this simple picture, there are many aspects of the chemistry in evolved stars that are not understood. Is IRC+10216 truly representative of carbon-rich AGB envelopes? What elemental abundances result from AGB nucleosynthesis? What is the contribution of oxygen-rich shells to molecule formation? What is the fate of clumps of gas and dust found in old PN? What is the original source of carbon–carbon bonds? Such questions have implications ranging from the origin of the elements to the origin of life, from galactic chemical evolution to the death of stars. Our picture of the chemistry in circumstellar shells is only beginning to emerge. More observational data on a wider sample of these objects, PN, and even diffuse clouds are needed, as well as the laboratory spectroscopy and theoretical modeling that support this tantalizing aspect of astrochemistry.
Supplementary Material
Acknowledgments
I thank N. J. Woolf, A. J. Apponi, D. T. Halfen, and K. Xilouris for interesting discussions; the referees, in particular T. W. Hartquist, for useful comments; the staff of the Arizona Radio Observatory; the National Radio Astronomy Observatory for loan of an Atacama Large Millimeter Array Band 6 mixer; and M. Benson for preparation of the manuscript. This research was supported by the National Aeronautics and Space Administration through the Astrobiology Institute under Cooperative Agreement CAN-02-OSS-02 issued through the Office of Space Science.
Glossary
Abbreviations
- AGB
asymptotic giant branch
- RSG
red supergiant
- LTE
local thermodynamic equilibrium
- PN
planetary nebula(e)
- PPN
protoplanetary nebula(e)
- VYCMa
VY Canis Majoris.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Willson L. A. Annu. Rev. Astron. Astrophys. 2000;38:573–611. [Google Scholar]
- 2.van Loon J. T., Cioni M. R. L., Zijlstra A. A., Loup C. Astron. Astrophys. 2005;438:273–289. [Google Scholar]
- 3.Marvel K. B. Astron. J. 2005;130:261–268. [Google Scholar]
- 4.Herwig F. Annu. Rev. Astron. Astrophys. 2005;43:435–479. [Google Scholar]
- 5.Forestini M., Charbonnel C. Astron. Astrophys. 1997;123(Suppl.):241–272. [Google Scholar]
- 6.Lattanzio J., Forestini M. In: IAU Symposium 191, Asympototic Giant Branch Stars. Le Bertre T., Lébre A., Waelkens C., editors. Dordrecht, The Netherlands: Reidel; 1999. pp. 31–40. [Google Scholar]
- 7.Prantzos N., Aubert O., Audouze J. Astron. Astrophys. 1996;309:760–774. [Google Scholar]
- 8.Keady J. J., Hall D. N. B., Ridgway S. T. Astrophys. J. 1988;362:832–842. [Google Scholar]
- 9.Truong-Bach, Morris D., Nguyen-Q-Rieu Astron. Astrophys. 1991;249:435–442. [Google Scholar]
- 10.Tsuji T. Astron. Astrophys. 1973;23:411–431. [Google Scholar]
- 11.Glassgold A. E. Annu. Rev. Astron. Astrophys. 1996;34:241–278. [Google Scholar]
- 12.Millar T. J., Herbst E., Bettens R. P. A. Mon. Not. R. Astron. Soc. 2000;316:195–203. [Google Scholar]
- 13.Monnier J. D., Danchi W. C., Hale D. S., Tuthill P. G., Townes C. H. Astrophys. J. 2000;543:868–879. [Google Scholar]
- 14.Keady J. J., Ridgway S. T. Astrophys. J. 1993;406:199–214. [Google Scholar]
- 15.McCabe E. M., Smith R. C., Clegg R. E. S. Nature. 1979;281:263–266. [Google Scholar]
- 16.Lafont S., Lucas R., Omont A. Astron. Astrophys. 1982;106:201–213. [Google Scholar]
- 17.Lucas R., Guélin M., Kahane C., Audinos P., Cernicharo J. Astrophys. Space Sci. 1995;244:293–296. [Google Scholar]
- 18.Guélin M., Lucas R., Neri R. In: IAU Symposium 170, CO: Twenty-Five Years of Millimeter Spectroscopy. Latter W. B., Radford S., Jewell P. R., Mangum J. G., Bally J., editors. Dordrecht, The Netherlands: Kluwer; 1997. pp. 359–366. [Google Scholar]
- 19.Bieging J. H., Shaked S., Gensheimer P. D. Astrophys. J. 2000;543:897–921. [Google Scholar]
- 20.Duari D., Cherchneff I., Willacy K. Astron. Astrophys. 1999;341:L47–L50. [Google Scholar]
- 21.Meixner M., Campbell M. T., Welch W. J., Likkel L. Astrophys. J. 1998;509:392–414. [Google Scholar]
- 22.Skinner C. J., Meixner M., Barlow M. J., Collison A. J., Justtanont K., Blanco P., Pina R., Ball J. R., Keto E., Arens J. F., et al. Astron. Astrophys. 1997;328:290–310. [Google Scholar]
- 23.Highberger J. L., Savage C. S., Bieging J. H., Ziurys L. M. Astrophys. J. 2001;562:790–798. [Google Scholar]
- 24.Justtanont K., Barlow M. J., Tielens A. G. G. M., Hollenbach D., Latter W. B., Liu X.-W., Sylvester R. J., Cox P., Nguyen-Q-Rieu, Skinner C. J. Astron. Astrophys. 2000;360:1117–1125. [Google Scholar]
- 25.Cox P., Maillard J.-P., Huggins P. J., Forveille T., Simons D., Guilloteau S., Rigaut F., Bachiller R., Omont A. Astron. Astrophys. 1997;321:907–920. [Google Scholar]
- 26.Sánchez Contreras C., Sahai R., Gil De Paz A. Astrophys. J. 2002;578:269–289. [Google Scholar]
- 27.Herpin F., Cernicharo J. Astrophys. J. 2000;530:L129–L132. doi: 10.1086/312507. [DOI] [PubMed] [Google Scholar]
- 28.Schuster M. T., Humphreys R. M., Marengo M. Astron. J. 2006;131:603–611. [Google Scholar]
- 29.Bujarrabal V., Alcolea J. Astron. Astrophys. 1991;251:536–548. [Google Scholar]
- 30.Humphreys R. M., Davidson K., Ruch G., Wallerstein G. Astron. J. 2005;129:492–510. [Google Scholar]
- 31.Lipscy S. J., Jura M., Reid M. J. Astrophys. J. 2005;626:439–445. [Google Scholar]
- 32.Cernicharo J., Guélin M., Kahane C. Astron. Astrophys. 2000;142(Suppl.):181–215. [Google Scholar]
- 33.Ridgway S., Keady J. J. Astrophys. J. 1988;326:843–858. [Google Scholar]
- 34.Kwok S. Nature. 2004;430:985–991. doi: 10.1038/nature02862. [DOI] [PubMed] [Google Scholar]
- 35.Ziurys L. M., Savage C., Highberger J. L., Apponi A. J., Guélin M., Cernicharo J. Astrophys. J. 2002;564:L45–L48. [Google Scholar]
- 36.Ziurys L. M., Friberg P., Irvine W. M. Astrophys. J. 1989;343:201–207. doi: 10.1086/167696. [DOI] [PubMed] [Google Scholar]
- 37.Ziurys L. M. Astrophys. J. 1991;379:260–266. [Google Scholar]
- 38.Cernicharo J., Guélin M. Astron. Astrophys. 1987;183:L10–L12. [Google Scholar]
- 39.Ziurys L. M., Apponi A. J., Phillips T. G. Astrophys. J. 1994;433:729–732. [Google Scholar]
- 40.Kawaguchi K., Kagi E., Hirano T., Takano S., Saito S. Astrophys. J. 1993;406:L39–L42. [Google Scholar]
- 41.Ziurys L. M., Apponi A. J., Guélin M., Cernicharo J. Astrophys. J. 1995;445:L47–L50. [Google Scholar]
- 42.Guélin M., Lucas R., Cernicharo J. Astron. Astrophys. 1993;280:L19–L22. [Google Scholar]
- 43.Petrie S. Mon. Not. R. Astron. Soc. 1996;282:807–819. [Google Scholar]
- 44.Olofsson H. In: The Dusty and Molecular Universe. Wilson A., editor. Noordwijk, The Netherlands: European Space Agency; 2005. pp. 223–228. [Google Scholar]
- 45.Woods P. M., Schöier F. L., Nyman L.-Å., Olofsson H. Astron. Astrophys. 2003;402:617–634. [Google Scholar]
- 46.Bujarrabal V., Fuente A., Omont A. Astron. Astrophys. 1994;285:247–271. [Google Scholar]
- 47.Highberger J. L., Ziurys L. M. Astrophys. J. 2003;597:1065–1069. [Google Scholar]
- 48.Pardo J. R., Cernicharo J., Goicoechea J. R. Astrophys. J. 2005;628:275–282. [Google Scholar]
- 49.Olofsson H., Lindqvist M., Winnberg A., Nyman L.-Å., Nguyen-Q-Rieu Astron. Astrophys. 1991;245:611–615. [Google Scholar]
- 50.Sánchez Contreras C., Bujarrabal V., Alcolea J. Astron. Astrophys. 1997;327:689–698. [Google Scholar]
- 51.Sánchez Contreras C., Bujarrabal V., Neri R., Alcolea J. Astron. Astrophys. 2000;357:651–660. doi: 10.1051/0004-6361/201629288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balick B., Frank A. Annu. Rev. Astron. Astrophys. 2002;40:439–486. [Google Scholar]
- 53.Young K., Cox P., Huggins P. J., Forveille T., Bachiller R. Astrophys. J. 1999;522:387–396. [Google Scholar]
- 54.Bachiller R., Forveille T., Huggins P. J., Cox P. Astron. Astrophys. 1997;324:1123–1134. [Google Scholar]
- 55.Huggins P. J., Bachiller R., Planesas P., Forveille T., Cox P. Astrophys. J. 2005;160(Suppl.):272–285. [Google Scholar]
- 56.Dyson J. E., Hartquist T. W., Pettini M., Smith L. J. Mon. Not. R. Astron. Soc. 1989;241:625–630. [Google Scholar]
- 57.Hartquist T. W., Dyson J. E. Astron. Astrophys. 1997;319:589–592. [Google Scholar]
- 58.Howe D. A., Hartquist T. W., Williams D. A. Mon. Not. R. Astron. Soc. 1994;271:811–816. [Google Scholar]
- 59.Liu X.-W., Barlow M. J., Dalgarno A., Tennyson J., Lim T., Swinyard B. M., Cernicharo J., Cox P., Baluteau J.-P., Pequignot D., et al. Mon. Not. R. Astron. Soc. 1997;290:L71–L75. [Google Scholar]
- 60.Hasegawa T., Volk K., Kwok S. Astrophys. J. 2000;532:994–1005. [Google Scholar]
- 61.Milam S. N., Xilouris K., Woolf N. J., Ziurys L. M. Astrophys. J. 2006 in press. [Google Scholar]
- 62.Liszt H. S., Lucas R., Pety J. Astron. Astrophys. 2006;448:253–259. [Google Scholar]
- 63.Lucas R., Liszt H. S. Astron. Astrophys. 2002;384:1054–1061. [Google Scholar]
- 64.Van Dishoeck E. F., Black J. H. Astrophys. J. 1986;62(Suppl.):109–145. [Google Scholar]
- 65.Horn A., Møllendal H., Sekiguchi O., Uggerud E., Roberts H., Herbst E., Viggiano A. A., Fridgen T. D. Astrophys. J. 2004;611:605–614. [Google Scholar]
- 66.Halfen D. T., Apponi A. J., Woolf N. J., Polt R., Ziurys L. M. Astrophys. J. 2006;639:237–245. [Google Scholar]
- 67.Milam S. N., Savage C., Brewster M. A., Ziurys L. M., Wyckoff S. Astrophys. J. 2005;634:1126–1132. [Google Scholar]
- 68.Savage B. D., Sembach K. R. Annu. Rev. Astron. Astrophys. 1996;34:279–330. [Google Scholar]
- 69.Pizzarello S., Cooper G., Flynn G. In: Meterorites and the Early Solar System II. Lauretta D. S., McSween H. Y., editors. Tucson: Univ. of Arizona Press; 2006. pp. 9008–9039. [Google Scholar]
- 70.Anders E. Nature. 1989;342:255–257. doi: 10.1038/342255a0. [DOI] [PubMed] [Google Scholar]
- 71.Chyba C. F., Sagan C. Nature. 1992;355:125–132. doi: 10.1038/355125a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.