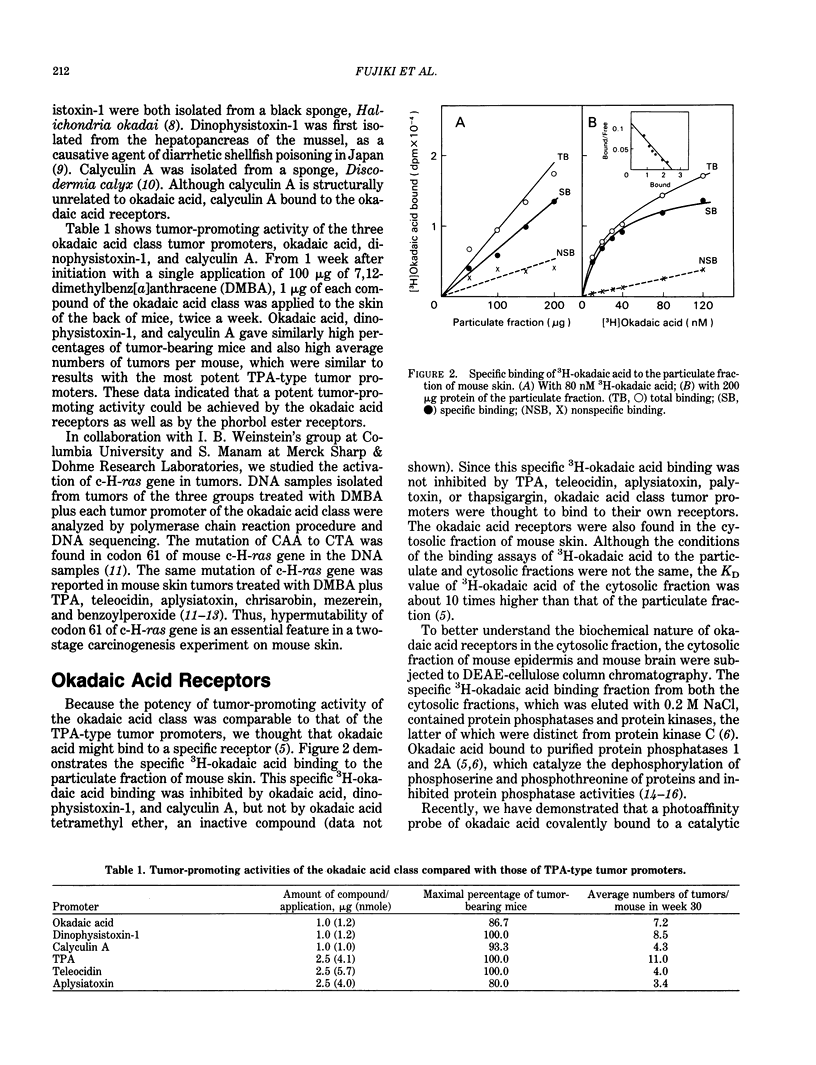
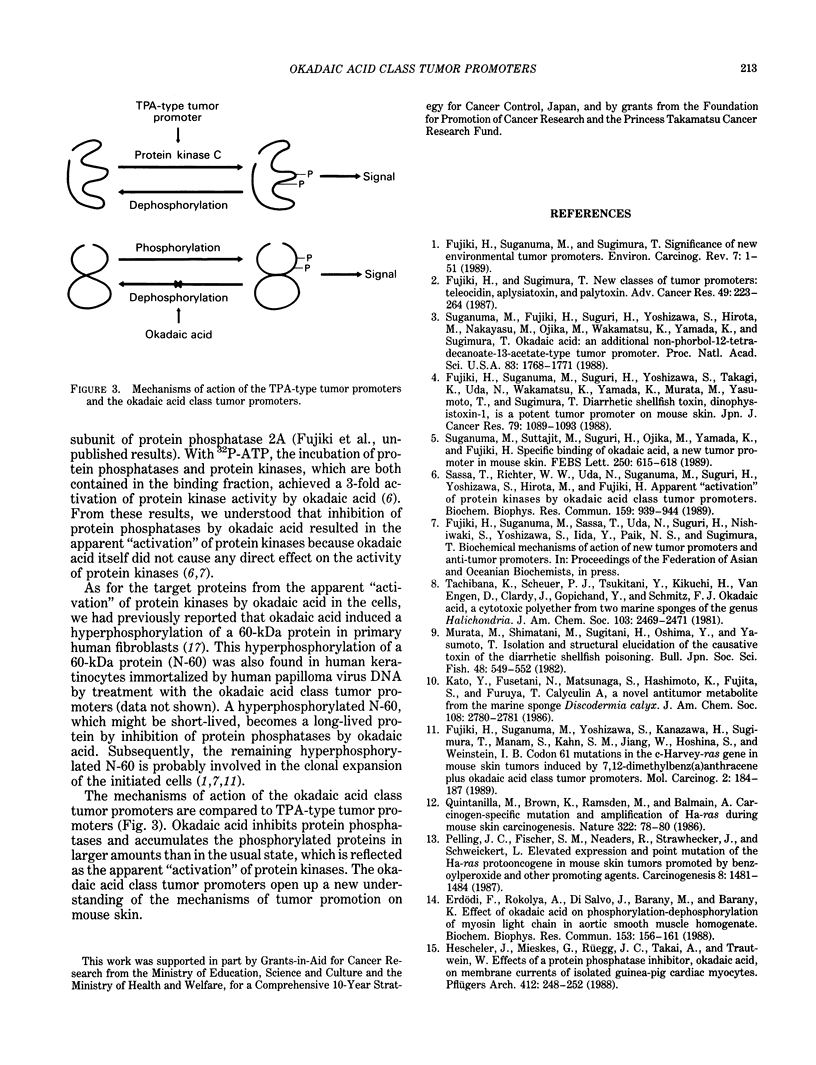
Abstract
Okadaic acid, dinophysistoxin-1 (35-methylokadaic acid), and calyculin A are the okadaic acid class of non-12-O-tetradecanoylphorbol-13-acetate (TPA)-type tumor promoters, which do not bind to the phorbol ester receptors in cell membranes or activate protein kinase C in vitro. They have potent tumor-promoting activities on mouse skin, as strong as TPA-type tumor promoters, such as TPA, teleocidin, and aplysiatoxin. DNA samples isolated from tumors induced by dimethylbenz[alpha]anthracene and each of the okadaic acid class tumor promoters had the same mutation at the second nucleotide of codon 61 (CAA to CTA) in the c-H-ras gene. Okadaic acid receptors, protein phosphatases 1 and 2A, are present in the particulate as well as cytosolic fractions of various mouse tissues. The apparent "activation" of protein kinases by the okadaic acid class tumor promoters, after their incubation with 32P-ATP, protein kinases, and protein phosphatases, was observed. This activation was caused by inhibition of protein phosphatases 1 and 2A by the okadaic acid class tumor promoters. Treatment of primary human fibroblasts and human keratinocytes with the okadaic acid class tumor promoters induced the hyperphosphorylation of a 60-kDa protein in nuclear and cytosolic fractions, due to the inhibition of protein phosphatases. The 60-kDa protein is a proteolytic fragment of nucleolin, a major nonhistone protein and is designated as "N-60." The mechanisms of action of the okadaic acid class tumor promoters are discussed with emphasis on the inhibition of protein phosphatase activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erdödi F., Rokolya A., Di Salvo J., Bárány M., Bárány K. Effect of okadaic acid on phosphorylation-dephosphorylation of myosin light chain in aortic smooth muscle homogenate. Biochem Biophys Res Commun. 1988 May 31;153(1):156–161. doi: 10.1016/s0006-291x(88)81202-6. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Suganuma M., Suguri H., Yoshizawa S., Takagi K., Uda N., Wakamatsu K., Yamada K., Murata M., Yasumoto T. Diarrhetic shellfish toxin, dinophysistoxin-1, is a potent tumor promoter on mouse skin. Jpn J Cancer Res. 1988 Oct;79(10):1089–1093. doi: 10.1111/j.1349-7006.1988.tb01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H., Suganuma M., Yoshizawa S., Kanazawa H., Sugimura T., Manam S., Kahn S. M., Jiang W., Hoshina S., Weinstein I. B. Codon 61 mutations in the c-Harvey-ras gene in mouse skin tumors induced by 7,12-dimethylbenz[a]anthracene plus okadaic acid class tumor promoters. Mol Carcinog. 1989;2(4):184–187. doi: 10.1002/mc.2940020403. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Sugimura T. New classes of tumor promoters: teleocidin, aplysiatoxin, and palytoxin. Adv Cancer Res. 1987;49:223–264. doi: 10.1016/s0065-230x(08)60799-x. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Sim A. T., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. G. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989 Jan 5;337(6202):78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Mieskes G., Rüegg J. C., Takai A., Trautwein W. Effects of a protein phosphatase inhibitor, okadaic acid, on membrane currents of isolated guinea-pig cardiac myocytes. Pflugers Arch. 1988 Aug;412(3):248–252. doi: 10.1007/BF00582504. [DOI] [PubMed] [Google Scholar]
- Issinger O. G., Martin T., Richter W. W., Olson M., Fujiki H. Hyperphosphorylation of N-60, a protein structurally and immunologically related to nucleolin after tumour-promoter treatment. EMBO J. 1988 Jun;7(6):1621–1626. doi: 10.1002/j.1460-2075.1988.tb02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelling J. C., Fischer S. M., Neades R., Strawhecker J., Schweickert L. Elevated expression and point mutation of the Ha-ras proto-oncogene in mouse skin tumors promoted by benzoyl peroxide and other promoting agents. Carcinogenesis. 1987 Oct;8(10):1481–1484. doi: 10.1093/carcin/8.10.1481. [DOI] [PubMed] [Google Scholar]
- Quintanilla M., Brown K., Ramsden M., Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986 Jul 3;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Sassa T., Richter W. W., Uda N., Suganuma M., Suguri H., Yoshizawa S., Hirota M., Fujiki H. Apparent "activation" of protein kinases by okadaic acid class tumor promoters. Biochem Biophys Res Commun. 1989 Mar 31;159(3):939–944. doi: 10.1016/0006-291x(89)92199-2. [DOI] [PubMed] [Google Scholar]
- Suganuma M., Suttajit M., Suguri H., Ojika M., Yamada K., Fujiki H. Specific binding of okadaic acid, a new tumor promoter in mouse skin. FEBS Lett. 1989 Jul 3;250(2):615–618. doi: 10.1016/0014-5793(89)80807-5. [DOI] [PubMed] [Google Scholar]


