Fig. 6.
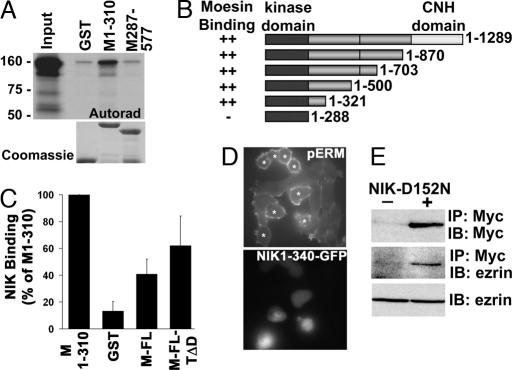
NIK 1-310 binds the FERM domain of moesin and is sufficient to phosphorylate ERM proteins in cells. (A Upper) Autoradiogram of binding assay with full-length NIK translated in vitro in the presence of [35S]methionine and GST and GST-moesin FERM domain (M1-310) and C terminus (M287-577) is shown. (A Lower) Coomassie-stained fusion proteins are shown. (B) NIK binding to the indicated fusion proteins relative to binding GST-M1-310 is shown. (C) Schematic representation of full-length (resides 1–1289) and C-terminal-truncated NIK used in binding assays is shown. ++, domains with comparable binding to GST-moesin 1–310. (D) Expression of an EGFP fusion of truncated NIK-1-340 is sufficient to increase phosphorylation of endogenous ERM proteins in CCL39 fibroblasts. Phosphorylation of ERM proteins was determined by immunolabeling with anti-pERM antibody. *, cells expressing NIK-1-340-EGFP are identified by fluorescence. (E) Ezrin coprecipitates with Myc-tagged kinase-inactive NIK (mNIK-D152N). (Top and Middle) Myc immunoprecipitates from lysates of CCL39 fibroblasts transfected with empty vector (−) or vector containing Myc-NIK-D152N (+) were probed with antibodies against Myc (Top) and ezrin (Middle). (Bottom) Ezrin in cell lysates was determined by immunoblotting. IP, immunoprecipitation; IB, immunoblotting.