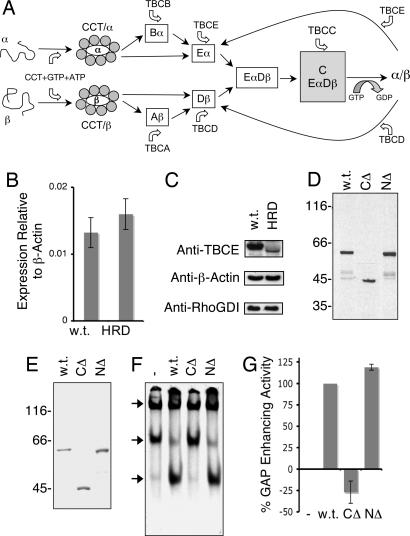
Fig. 1.
Expression and activity of wild-type and HRD mutant TBCE proteins. (A) Schematic representation of the tubulin heterodimer assembly pathway in higher eukaryotes (6, 9). (B) Expression levels of TBCE mRNA (relative to β-actin) in normal (w.t.) and compound heterozygote HRD patient-derived fibroblasts, determined by real-time PCR. (C) Abundance of TBCE protein determined by semiquantitative Western blotting by using an anti-TBCE antibody in postnuclear extracts of wild-type and HRD mutant (compound heterozygote) cells. Loading controls done with anti-β-actin and anti-RhoGDI antibodies are shown below. (D). Expression of wild-type (w.t.) and HRD mutant (CΔ, C371X; NΔ, deletion of nucleotides 66–67) forms of TBCE by transcription/translation in rabbit reticulocyte lysate. (E) Purification of recombinant wild-type and HRD mutant forms of TBCE expressed in insect sf9 cells. In D and E, location of molecular mass markers is shown on the left. (F) In vitro β-tubulin-folding assays (5, 6), in which bacterially expressed 35S-labeled urea-unfolded β-tubulin was suddenly diluted into a reaction containing the cytosolic chaperonin CCT, ATP, GTP, TBCC, and TBCD and either wild-type (w.t.) or compound heterozygous HRD mutant forms (NΔ, deletion of nucleotides 66–67) of TBCE. Reaction products were analyzed by nondenaturing gel electrophoresis. Arrows (top to bottom) denote the location of the CCT/β-tubulin binary complex, the β-tubulin/TBCD complex, and native tubulin heterodimers. (G) Relative tubulin GAP-enhancing activities (17) of compound heterozygous HRD mutant [C371X (CΔ) and deletion of nucleotides 66–67 (NΔ)] forms of TBCE protein relative to wild type (taken as 100%).