Abstract
Alpha-ketoglutarate dehydrogenase (α-KGDH) is a highly regulated enzyme, which could determine the metabolic flux through the Krebs cycle. It catalyses the conversion of α-ketoglutarate to succinyl-CoA and produces NADH directly providing electrons for the respiratory chain. α-KGDH is sensitive to reactive oxygen species (ROS) and inhibition of this enzyme could be critical in the metabolic deficiency induced by oxidative stress. Aconitase in the Krebs cycle is more vulnerable than α-KGDH to ROS but as long as α-KGDH is functional NADH generation in the Krebs cycle is maintained. NADH supply to the respiratory chain is limited only when α-KGDH is also inhibited by ROS. In addition being a key target, α-KGDH is able to generate ROS during its catalytic function, which is regulated by the NADH/NAD+ ratio. The pathological relevance of these two features of α-KGDH is discussed in this review, particularly in relation to neurodegeneration, as an impaired function of this enzyme has been found to be characteristic for several neurodegenerative diseases.
Keywords: alpha-ketoglutarate dehydrogenase, reactive oxygen species, mitochondria, oxidative stress, neurodegeneration
Alpha-ketoglutarate dehydrogenase (α-KGDH) is a Krebs cycle enzyme, which catalyses the non-equilibrium reaction converting α-ketoglutarate, coenzyme A and NAD+ to succinyl-CoA, NADH and CO2, requiring thiamine pyrophosphate as a cofactor. α-KGDH is not simply one of the enzymes of the Krebs cycle. There are a number of features that makes this enzyme distinct from other enzymes important in the bioenergetic processes. First of all, it is highly regulated and is the primary site of control of the metabolic flux through the Krebs cycle (Hansford 1980). The mammalian enzyme is inhibited by its end products, succinyl-CoA and NADH (Garland 1964; Smith et al. 1974). Regulation of α-KGDH is complex involving ATP/ADP ratio, NADH/NAD+ ratio, calcium and the substrate availability in mitochondria; therefore, it is also related to the activity of NAD+-isocitrate dehydrogenase (ICDH; Hansford 1980). The apparent Km (Michaelis constant) of the enzyme for α-ketoglutarate in the mitochondria is 0.67 mM (Hansford 1980).
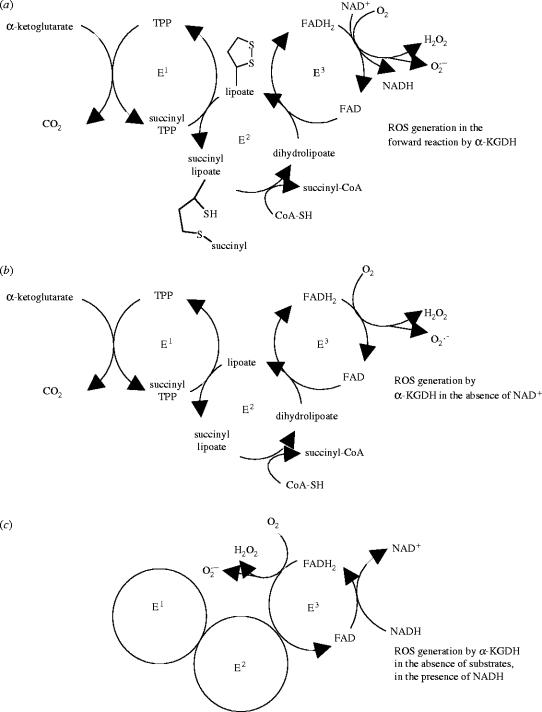
α-KGDH is a complex enzyme consisting of multiple copies of three subunits (see figure 2), a thiamine pyrophosphate-dependent dehydrogenase (E1), dihydrolipoamide succinyltransferase (E2) and dihydrolipoamide dehydrogenase (E3), the latter being a flavoprotein (Koike et al. 1974; Koike & Koike 1976).
Figure 2.
Generation of reactive oxygen species by α-KGDH. Subunit composition of α-KGDH and generation of ROS in the physiological forward reaction of the enzyme, when substrates and cofactors and oxygen are present (a). ROS generation in the forward reaction by α-KGDH is enhanced in the absence of NAD+ (b). ROS generation by the E3 subunit induced by NADH in the absence of substrates (c). E1, α-ketoglutarate dehydrogenase; E2, dihydrolipoamide succinyltransferase; E3, dihydrolipoamide dehydrogenase; TPP, thiamine pyrophosphate.
Calcium plays a key role in activating three mitochondrial dehydrogenases, pyruvate dehydrogenase (PDH), isocitrate dehydrogenase and α-KGDH in the 0.1–10 μM range (Denton et al. 1978; Denton & McCormack 1980) and by this, in adjusting the rate of metabolism to an increased demand. Activation of these dehydrogenases could increase the supply of NADH to the respiratory chain and, indeed, an increased reduction of NAD+ by α-ketoglutarate was monitored in rat heart mitochondria (Hansford & Castro 1981). The first evidence that an increase in the intracellular calcium concentration is able to activate mitochondrial dehydrogenases resulting in an increase in the NADH level in intact cells was provided by Duchen (1992) and Pralong et al. (1992), demonstrating that NADH level is increased in cells in a calcium-dependent manner in response to depolarization. Calcium lowers the apparent Km of α-KGDH for α-ketoglutarate (McCormack & Denton 1979), this mechanism being different from that underlying the activation of PDH, where calcium promotes the dephosphorylation of the enzyme (Denton et al. 1972; Pettit et al. 1972). Activation of dehydrogenases occurs at relatively low calcium concentrations (less than 20 μM). Calcium, in high concentrations (greater than or equal to 100 μM), however, inhibits α-KGDH (Lai & Cooper 1986), ICDH (Bulos et al. 1984) and PDH (Sheu et al. 1985). This effect could significantly contribute to the deleterious effects of high calcium concentration in neurons under pathological conditions.
α-KGDH could be a crucial target of reactive oxygen species (ROS) in cells and, being an important regulatory site in the mitochondrial metabolism, could play a key role in the bioenergetic deficit evolving in oxidative stress. On the other hand, it has been revealed recently that the enzyme itself is able to generate ROS, therefore could contribute to the induction of oxidative stress. This review will focus on these two features of α-KGDH and discuss the relevance of α-KGDH in oxidative stress, concentrating mainly on the brain enzyme and its relation to neurodegeneration.
Early studies performed with brain mitochondria demonstrated that the activity of α-KGDH was the lowest among the Krebs cycle enzymes (Lai et al. 1977), indicating that, similarly to peripheral tissues (Hansford 1980), it could also be a rate-limiting enzyme in the brain regulating the flux through the cycle. The regional distribution of α-KGDH in the brain is not uniform but follows the pattern of other citric acid cycle and glycolytic enzymes (Leong et al. 1981; Leong & Clark 1984); the activity is highest in cerebral cortex and lowest in the phylogenetically older parts of the brain (Lai & Cooper 1986). The properties of the brain α-KGDH appear to differ, in many respects, from those of the enzyme originating from non-neuronal tissues. For instance, brain enzyme is inhibited by ammonia, whereas the enzyme in isolated rat heart or purified from bovine heart was much less sensitive to inhibition (Lai & Cooper 1986). Inhibitors of the bovine heart enzyme, β-methylene-d,l-aspartate or d,l-vinylglycine, were without effect on the brain mitochondrial α-KGDH but l-aspartate, which did not influence the activity of the purified bovine heart α-KGDH, significantly inhibited the enzyme in rat brain mitochondria (Lai & Cooper 1986).
1. α-KGDH is a crucial target of reactive oxygen species in the mitochondria
Oxidative stress involves an increased production and/or a decreased elimination of ROS in cells and has been shown to contribute to the cell damage caused by ischaemia/reperfusion, trauma, ageing and several neurodegenerative diseases (Halliwell & Gutteridge 1984, 1985; Cao et al. 1988; Schmidley 1990; Halliwell 1992; Hall et al. 1993; Olanow 1993; Siesjo et al. 1995). The effect of ROS (superoxide, hydrogen peroxide and hydroxyl radical) could be damaging on virtually all cellular components but membrane lipids are particularly sensitive to free radicals due to the presence of polyunsaturated fatty acids, which preferentially undergo lipid peroxidation (Halliwell & Gutteridge 1984, 1985). The fragmentation products of lipid peroxidation are highly toxic aldehydes, of which 4-hydroxy-2-nonenal (HNE) is the major and probably the most reactive product (Esterbauer et al. 1991). Exposure of cardiac mitochondria to HNE resulted in a reduced α-KGDH activity and an inhibition of the NADH-linked state three respiration (Humphries et al. 1998). Besides α-KGDH only PDH was inhibited by HNE; other mitochondrial dehydrogenases and complexes of the respiratory chain were unaltered by HNE (Humphries et al. 1998). Investigation of the effect of HNE on isolated α-KGDH and PDH revealed that the target of HNE is lipoic acid bound covalently to the E2 subunit of both α-KGDH and PDH (Humphries & Szweda 1998).
α-KGDH is also inactivated by other reactive products. Exposure of soleus muscle to the superoxide donor xanthine and xanthine oxidase inhibited the activity of α-KGDH in muscle extracts but nitric oxide alone had no effect (Andersson et al. 1998). However, superoxide and nitric oxide together, which generate peroxynitrate, were more effective than superoxide alone, in inactivating the enzyme (Andersson et al. 1998). Consistent with this, isolated α-KGDH as well as the enzyme present in the microglia were inactivated by peroxynitrate and nitric oxide and proved to be at least 10-times more sensitive to these agents than another mitochondrial enzyme, glutamate dehydrogenase (Park et al. 1999). Peroxynitrate nitrated tyrosine residues of the α-KGDH subunits and reduced the immunoreactivity to antibodies against the E1 and E2 but not the E3 subunits (Park et al. 1999).
The toxic amyloid-β-peptide, which accumulates in the plaques in Alzheimer's disease, increases oxidative stress in cultured neurons (Harris et al. 1995; Mark et al. 1997) and directly inhibits PDH and α-KGDH in brain mitochondria (Casley et al. 2002), pointing to the possible involvement of these enzymes in the defective energy metabolism characteristic to the affected brain regions in Alzheimer's disease.
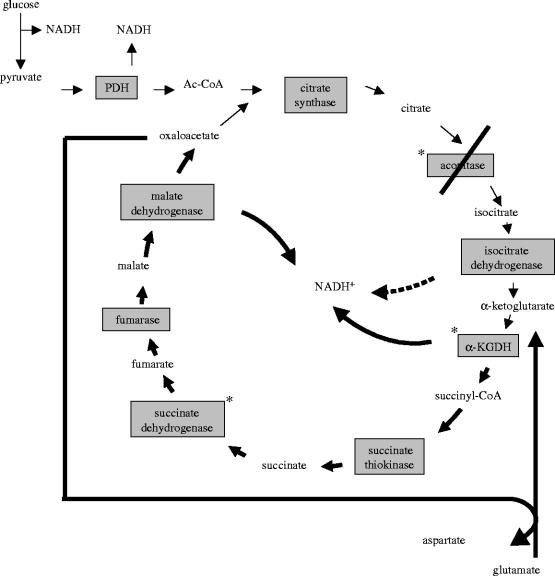
Our group, using isolated nerve terminals (synaptosomes), demonstrated first that α-KGDH is sensitive to H2O2 (Chinopoulos et al. 1999; Tretter & Adam-Vizi 2000), and this was confirmed for fibroblasts (Gibson et al. 2000) and cardiomyocytes (Nulton-Persson & Szweda 2001). α-KGDH in nerve terminals exposed to 0.1 or 0.5 mM H2O2 for 10 min was inhibited by 40 and 50%, respectively (Chinopoulos et al. 1999). In a detailed study addressing the sensitivity of the Krebs cycle enzymes to oxidative stress, we found that three enzymes were inhibited by H2O2: aconitase, α-KGDH and succinate dehydrogenase, but the vulnerability of these enzymes to H2O2 and the metabolic consequences of their impaired function were markedly different (Tretter & Adam-Vizi 2000). The least significant was the effect of H2O2 on succinate dehydrogenase, which is not a rate-limiting enzyme in the Krebs cycle and was only inhibited by ca 30% in the presence of the highest concentration of H2O2 (0.5 mM). In contrast, aconitase showed a high sensitivity to H2O2, being completely inactivated at already very small H2O2 concentrations (less than 50 μM). In fact, aconitase was the only enzyme which could be inhibited by 100% upon acute exposure to H2O2. Yet, the impaired bioenergetic consequences in the Krebs cycle under the early phase of an H2O2-induced oxidative stress are not due to inhibition of aconitase but rather that of α-KGDH. The key question here is whether the overall function of the Krebs cycle, as shown by the generation of NADH, is impaired by H2O2 and if so, which enzyme(s) limits the generation of NADH under oxidative stress.
For the clarification of the impact and the metabolic consequences of the inhibition of α-KGDH under oxidative stress it is important first to consider the role of aconitase in oxidative stress. Gardner & Fridovich (1992) and Gardner et al. (1995) reported first that mammalian aconitase was inhibited by superoxide and later it has been shown that nitric oxide (Andersson et al. 1998), peroxynitrate (Andersson et al. 1998) and, as shown by our group, H2O2 (Chinopoulos et al. 1999; Tretter & Adam-Vizi 2000) also inhibited this enzyme, most likely due to interaction with the iron–sulphur clusters. Generally, iron–sulphur centres of proteins are primary sites for ROS to attack mitochondrial enzymes and this may be the mechanism by which succinate dehydrogenase is also inhibited by ROS. The extreme sensitivity of aconitase to ROS allows the measurement of this enzyme to be an indicator of superoxide (Patel et al. 1996) and H2O2 production (Tretter & Adam-Vizi 2000; Sipos et al. 2003a,b) in cells.
Aconitase was inhibited in cortical cell culture treated with excitotoxic concentrations of NMDA or kainate and this was prevented by the cell-permeable superoxide dismutase mimetic manganese(III) 5,10,15,20-tetra(4-pyridyl)-21H,23H-porphine chloride terakis (methochloride) (MnTBAP) indicating that the active species was superoxide (Patel et al. 1996). Conversion of citrate to isocitrate catalysed by aconitase is thought to be a near-equilibrium reaction in vivo, therefore not believed to be a regulatory site in the Krebs cycle. However, inhibition of aconitase could lower the flux through the cycle due to accumulation of citrate and a consequent inhibition of citrate synthase, or could block the flux when inhibited by 100%. Patel et al. (1996) indeed found an inactivation of aconitase that paralleled the amount of cell death after exposure of cortical cells to excitotoxic insults. However, when we addressed specifically the question whether inhibition of aconitase limits NADH production in the Krebs cycle, it was found that in the early period of oxidative stress the function of the cycle is dependent on the inhibition of α-KGDH and not that of aconitase (Tretter & Adam-Vizi 2000). Parallel measurements of aconitase and NAD(P)H generation revealed that aconitase could be inhibited by 100% in the presence of H2O2 (up to 50 μM concentration) without any change in the maximum available NAD(P)H level in nerve terminals. NAD(P)H level decreased only at higher H2O2 concentrations (greater than or equal to 50 μM), when aconitase was already completely inactivated, therefore no correlation was seen between the inhibition of this enzyme and the impaired NAD(P)H generation in the Krebs cycle. At higher H2O2 concentrations, α-KGDH was also inhibited and this showed a correlation with a fall in the NAD(P)H level in nerve terminals (Tretter & Adam-Vizi 2000). A clear conclusion offered by these results is that though α-KGDH is less sensitive to H2O2 than aconitase, because it is inhibited at higher oxidant concentrations and is never inactivated by 100%, yet this is the key enzyme that limits the generation of NAD(P)H in the Krebs cycle under acute exposure to oxidative stress. α-KGDH, as discussed above, is one of the key regulatory enzymes, which, together with citrate synthase and isocitrate dehydrogenase, is thought to determine the overall rate of the Krebs cycle (Cooney et al. 1981; McCormack et al. 1990; Moreno-Sanchez et al. 1990). Nonetheless, it has to be resolved how the Krebs cycle can function and generate NAD(P)H when one of the enzymes, aconitase, even though not rate-limiting, is completely blocked. The explanation for this apparent contradiction is that when the input to Krebs cycle from pyruvate is inhibited due to the block of aconitase, a segment of the cycle between α-ketoglutarate and oxaloacetate can still function if glutamate is available and feeds the cycle via α-ketoglutarate (figure 1). This segment of the Krebs cycle has been suggested to function in the absence of glucose, when most likely glutamate is converted to α-ketoglutarate (Yudkoff et al. 1994; Erecinska et al. 1996). In this respect oxidative stress, in a phase when aconitase is already completely blocked but α-KGDH is still functional, is similar to glucose-free condition. We found that the amount of glutamate present in nerve terminals decreased when aconitase was fully blocked by H2O2 (Tretter & Adam-Vizi 2000). Fuelling the Krebs cycle from glutamate via α-ketoglutarate is possible by transamination due to the presence of aspartate aminotransferase, rather than glutamate dehydrogenase, which has limited activity in nerve terminals (Cheeseman & Clark 1988; Yudkoff et al. 1994). This rescue route could explain that in the early phase of an oxidative stress, NAD(P)H generation in the Krebs cycle is maintained in spite of a blockage of aconitase (figure 1). When at a higher degree of oxidative stress α-KGDH is also inhibited, NAD(P)H generation becomes limited (Chinopoulos et al. 1999; Tretter & Adam-Vizi 2000). The oxidative stress-induced inhibition of α-KGDH is critical because it decreases the provision of NAD(P)H for complex I, thus decreasing the rate of the respiratory chain. Under this condition, the ATP production decreases in nerve terminals (Tretter et al. 1997) and the membrane potential of mitochondria could be maintained only with the contribution of the F0F1-ATPase working in reverse and hydrolysing ATP. This was apparent from ΔΨm measurements, which revealed that as long as the F0F1-ATPase was active ΔΨm was maintained under H2O2-induced oxidative stress, but when the enzyme was inhibited by oligomycin, ΔΨm decreased indicating that the respiratory chain was insufficient to maintain ΔΨm (Chinopoulos et al. 1999). Nerve terminals, with an impaired α-KGDH activity, produce less ATP by ca 30% in the early phase of oxidative stress (Tretter et al. 1997), but this is still adequate to maintain the resting function of the ATP-driven ion pumps in the plasma membrane. When oxidative stress is superimposed on some other burden such as a [Na+]i load occurring during excessive stimulation of glutamate receptors or in ischaemia/reperfusion, the inability of mitochondria to meet an additional ATP demand, mainly due to inhibition of α-KGDH, becomes apparent and the [Na+] and [Ca2+] gradients across the plasma membrane (Chinopoulos et al. 2000) as well as the mitochondrial membrane potential rapidly collapse (Scanlon & Reynolds 1998).
Figure 1.
Metabolic flux in the Krebs cycle during oxidative stress when aconitase is completely inactivated. * Enzymes inhibited by H2O2. When aconitase, the most sensitive enzyme, is inhibited by 100% but α-KGDH is still functional in the presence of H2O2, a segment of the Krebs cycle (bold arrows) is maintained by glutamate, which is converted to α-ketoglutarate via transamination. This segment is inhibited only when α-KGDH is also inhibited by ROS. PDH, pyruvate dehydrogenase.
α-KGDH was found to be inhibited in cardiac mitochondria isolated after myocardial ischaemia and reperfusion (Lucas & Szweda 1999). The reperfusion-induced inactivation of α-KGDH involved the oxidative damage of lipoic acid essential for the catalytic activity of the enzyme and was specific; the activities of other dehydrogenases were not influenced by ischemia/reperfusion (Sadek et al. 2002). It has been suggested that free radicals are crucial in mediating reperfusion-induced injuries (Ambrosio et al. 1991, 1993; Blasig et al. 1995; Bolli et al. 1988) but the nature of radicals acting under this condition has not been defined yet. The effect of H2O2 on α-KGDH might be significant during reperfusion for it is formed in a relatively high concentration (0.1 mM), as detected by microdialysis in the striatum during reperfusion following an ischaemic period (Hyslop et al. 1995).
Another factor that might play a role in the inhibition of α-KGDH during ischaemia/reperfusion could be Zn2+, which is present in elevated concentrations in cells under this condition (Tonder et al. 1990; Koh et al. 1996). It has been reported that Zn2+, in submicromolar concentrations, induces reversible inhibition of the purified α-KGDH and inhibits respiration in liver mitochondria, the α-ketoglutarate-dependent respiration showing the highest Zn2+-sensitivity (Brown et al. 2000). Zn2+ also inhibited glutamate dehydrogenase and PDH, but not succinate dehydrogenase or malate dehydrogenase (Brown et al. 2000). Zn2+ has been implicated in the pathogenesis of excitotoxic injury (Weiss et al. 2000; Dineley et al. 2003; Frederickson et al. 2004), another condition in which oxidative stress is a key event (Coyle & Puttfarcken 1993; Lafon-Cazal et al. 1993; Reynolds & Hastings 1995; Patel et al. 1996; Wang & Thayer 1996; White & Reynolds 1996). It has been demonstrated that Zn2+ interacts with lipoamide dehydrogenase and inhibits both the forward and the reverse reaction (see below) catalysed by the isolated enzyme (Gazaryan et al. 2002), pointing to the site of effect of Zn2+ on α-KGDH.
The susceptibility of α-KGDH to a variety of conditions somehow related to oxidative stress strongly suggests that the deficiency of this enzyme in oxidative stress could be crucial in the metabolic defects caused by ROS.
α-KGDH appears to be more sensitive to disturbed homeostatic factors than other enzymes. In post-mortem mouse brain samples, the activity of α-KGDH is quickly lost whereas the activity of another TPP-dependent enzyme, PDH, remains unaltered for at least 24 h (Gibson et al. 1988; Mizuno et al. 1990). Mastrogiacomo et al. (1993) found a correlation between the lactate level in the brain samples and the decrease in the activity of α-KGDH, which could indicate that α-KGDH is more sensitive to antemortem hypoxia than other enzymes involved in metabolism (Terwel et al. 1998). It is of note here that the pH optimum of the brain mitochondrial α-KGDH is very narrow, between 7.2 and 7.4, and at pH values below 7.2 or above 7.8 the activity of the enzyme markedly decreased (Lai & Cooper 1986). α-KGDH purified from pig heart showed a broader pH optimum, between pH 6.6 and 7.4 when the maximum activity of the enzyme was measured, but the Km of the enzyme was significantly increased in this pH range (McCormack & Denton 1979).
Sulphydryl groups which are critical to the catalytic activity of α-KGDH on the E2 subunit of the enzyme may be responsible for the increased vulnerability of the enzyme to ROS or nitric oxide, whereas tyrosine residues make this enzyme a target for peroxynitrate-induced nitration.
2. α-KGDH as a generator of oxidative stress
It is firmly believed that mitochondrial electron transport chain is a rich source of ROS (Boveris et al. 1972; Loschen et al. 1973). Large number of studies using different substrates supporting the respiration and different inhibitors of the respiratory complexes established that ROS can be produced at complex I (NADH ubiquinone oxidoreductase; Cadenas et al. 1977; Takeshige & Minakami 1979; Korshunov et al. 1997; Herrero & Barja 2000; Votyakova & Reynolds 2001; Liu et al. 2002; Starkov & Fiskum 2003; for review see Turrens 2003) and the Q-cycle of the respiratory chain (Boveris & Chance 1973; Loschen et al. 1974; Boveris et al. 1976; Turrens & Boveris 1980; Starkov & Fiskum 2001). With a critical view one has to admit that it is impossible to determine at present how and where in the respiratory chain ROS are generated in in situ mitochondria under resting condition, when during the cellular metabolism the respiratory chain receives electrons both from FADH2 and NADH. In situ mitochondria in isolated nerve terminals react with an increased ROS generation to an already small degree of complex I inhibition, which suggest that this could be physiologically more important than ROS production in response to complex III inhibition, which requires more than 70% inhibition of this complex (Sipos et al. 2003a).
Recently, the generally accepted idea that complexes of the respiratory chain are the ‘major’ site of ROS production in the mitochondria has been challenged. Independent studies have demonstrated that α-KGDH could be an important source of ROS (Starkov et al. 2004; Tretter & Adam-Vizi 2004).
Earlier it has been reported that flavoproteins, generally, can activate oxygen, resulting in the production of superoxide and/or hydrogen peroxide (Ballou et al. 1969; Chan & Bielski 1980; Kakinuma et al. 1987; Massey 1994; Zhang et al. 1998). Lipoamide dehydrogenase, the E3 subunit of α-KGDH, is a flavoenzyme, being responsible for the transfer of reducing equivalents from lipoate, which is bound to the E2 subunit, to NAD+, the final electron acceptor in the α-KGDH catalysed reaction (figure 2). Lipoamide dehydrogenase is not unique for α-KGDH, it is also a component of PDH and branched-chain ketoacid dehydrogenase. With dehydrogenases, generally, the flavoprotein-mediated ROS generation was found to be very slow with a primary product being poorly defined (Massey 1994). The E3 subunit of α-KGDH can be studied either as part of α-KGDH by using dihydrolipoate (plus NAD+) instead of α-ketoglutarate as substrate, or as an isolated enzyme with the same substrate. Under this condition dihydrolipoate (L(SH)2) is oxidized to lipoate . This reaction is reversible, in the presence of NADH, free lipoate is reduced to dihydrolipoate . It was reported that isolated lipoamide dehydrogenase generates hydrogen peroxide in the reverse reaction exhibited under a non-physiological condition, when in the presence of oxygen only NADH but no lipoate, the substrate, is present (Huennekens et al. 1955; Massey et al. 1969; Bando & Aki 1991; Gazaryan et al. 2002). In the lipoamide dehydrogenase reaction, superoxide was also produced and the ratio of superoxide and hydrogen peroxide generated in the reaction was found to be 1 : 9 (Bando & Aki 1991). Interestingly, it has been reported that Zn2+, which could inhibit α-KGDH (Brown et al. 2000), is able to stimulate the oxidase activity of the isolated lipoamide dehydrogenase (Gazaryan et al. 2002). In light of the report that Zn2+ is also able to induce ROS generation in the mitochondria (Sensi et al. 1999), it is possible that Zn2+-induced ROS generation by lipoamide dehydrogenase could be of pathological importance.
Recently, our group (Tretter & Adam-Vizi 2004) and Starkov et al. (2004) have demonstrated that α-KGDH, during the physiological catalytic function of the enzyme, generates hydrogen peroxide and to a smaller extent superoxide. H2O2 generation by α-KGDH was inhibited by 80% in the presence of NAD+ and accelerated by addition of NADH (Tretter & Adam-Vizi 2004). The most important regulator of the α-KGDH-mediated ROS generation appeared to be the NADH/NAD+ ratio. When this was increased the physiological catalytic function and the ROS generation by α-KGDH were clearly dissociated, the physiological reaction by the enzyme being inhibited and the ROS generation becoming dominant (Tretter & Adam-Vizi 2004). With the isolated enzyme, ROS generation by the enzyme working in the physiological direction could be distinguished from that attributed to a reverse mode of the enzyme. When the physiological substrates (α-ketoglutarate and CoA) are present, but NAD+, the physiological electron acceptor, is not available, electrons derived from the substrate oxidation reduce oxygen and produce ROS in the forward reaction. For the stimulation of ROS generation by NADH, no added substrates were required, only the presence of the enzyme and NADH was sufficient (Tretter & Adam-Vizi 2004). This clearly suggested that ROS is generated on the E3 subunit and for this electrons can be provided either by the oxidation of α-ketoglutarate involving the E1 and E2 subunits (forward, physiological direction) or by NADH in the non-physiological reverse reaction (figure 2).
α-KGDH-mediated ROS production demonstrated with the isolated enzyme could be significant in mitochondria as shown in studies performed with isolated rat brain mitochondria (Starkov et al. 2004), or intact synaptosomes where ROS generation in in situ mitochondria can be investigated (Tretter & Adam-Vizi 2004). In the experiments by Starkov et al. (2004) ROS production in isolated mitochondria respiring on different substrates correlated with the reduction level of mitochondrial NAD(P)H, with the exception of α-ketoglutarate, which supported the highest rate of ROS generation. In the presence of the complex I inhibitor, rotenone, the rate of H2O2 generation was three times higher with α-ketoglutarate than with succinate (Starkov et al. 2004), which might be consistent with the effect of an increased NADH/NAD+ ratio in the mitochondria under this condition.
In situ synaptic mitochondria present in isolated nerve terminals (synaptosomes) produce H2O2 in the presence of glucose under resting conditions, which can be detected by measuring H2O2 release from synaptosomes into the medium or following the activity of endogenous aconitase (Sipos et al. 2003a,b). α-Ketoglutarate, which is taken up by synaptosomes, could maintain the mitochondrial respiration and ATP production by directly fuelling α-KGDH (Shank & Campbell 1984; Willoughby et al. 1989). When α-ketoglutarate maintained the mitochondrial metabolism the rate of H2O2 release from synaptosomes was 2.5 times higher than that measured in the presence of glucose, and consistent with this, the activity of endogenous aconitase as an indicator of ROS generation was significantly decreased (Tretter & Adam-Vizi 2004). This observation strongly supports the suggestion that α-KGDH present in mitochondria in situ could generate ROS in significant amount to interact with aconitase, which is arranged in the close proximity of α-KGDH in the Krebs cycle.
In the study performed by Starkov et al. (2004), ROS formation by α-KGDH was also attributed to the E3 subunit as brain mitochondria prepared from heterozygous knock-out mice deficient in the E3 subunit produced significantly less H2O2 than mitochondria prepared from their littermate wild-type mice (Starkov et al. 2004). The rate of H2O2 generation in the presence of α-ketoglutarate in response to rotenone was also significantly reduced in mitochondria isolated from E3-deficient mice. This result together with our observation that an increase in the NADH/NAD+ ratio favours ROS generation by α-KGDH raises the possibility that generation of ROS by α-KGDH could contribute to the accumulation of ROS in mitochondria in response to inhibition of complex I. It is not possible to estimate, as yet, the extent of contribution of α-KGDH to the ROS generation in mitochondria, but it could be assumed that when complex I is inhibited, like in the case of Parkinson's disease (see below), the oxidative stress that is characteristic for the pathology of the disease (Beal 1996, 2001; Mizuno et al. 1998; Schapira 1999) is partly due to ROS generation by this enzyme. A further factor that makes this picture more intricate is that complex I itself is vulnerable to ROS (Zhang et al. 1990; Chinopoulos & Adam-Vizi 2001) and would be inhibited under oxidative stress creating a vicious cycle, eventually building up a large degree of oxidative stress.
Different radical species as side products in the 2-oxo acid dehydrogenase reaction was detected by spin trapping involving superoxide in the presence of oxygen and thiyl radical of the complex-bound dihydrolipoate in the absence of oxygen, and the latter was suggested to mediate the inactivation of the enzyme on the E1 subunit (Bunik & Sievers 2002). The physiological importance of this self-regulated inactivation occurring when the concentration of NAD+ is low in the mitochondria could be the sparing of 2-oxo acid and prevention of production of radical species, as proposed by Bunik (2003). Given the sensitivity of α-KGDH to ROS discussed earlier, it is feasible to suggest that the enzyme is also inactivated when radicals are produced by the enzyme itself in the immediate vicinity of the ROS-sensitive components. However assuming that this happens in vivo, it may indeed spare α-ketoglutarate but radicals could still be generated even when E1 or E2 subunits are inhibited. Conditions involving a decrease in NAD+ concentration in the mitochondria usually parallel an increase in NADH level, and ROS generation by α-KGDH induced by NADH, as shown in our study (Tretter & Adam-Vizi 2004), would require only the E3 subunit by which ROS generation could be maintained.
A beneficial aspect of ROS generation by α-KGDH in the physiological direction, when α-ketoglutarate is oxidized, could be also envisaged under specific pathological conditions. In particular, when reoxidation of NADH in the respiratory chain is impaired due to complex I deficiency it could be important to channel electrons to the respiratory chain through complex II and maintain ATP production. ROS generation by α-KGDH could enable the conversion of α-ketoglutarate to succinyl-CoA to a certain extent even when the physiological electron acceptor, NAD+, is not sufficiently available. At present this is only a hypothesis and it needs further clarification as to what extent ROS generation by α-KGDH could serve the maintenance of the flow through the Krebs cycle, thus being of use for bioenergetics, particularly when complex I is inhibited. It is also unclear at present to what extent the α-KGDH-mediated ROS generation could contribute to the accumulation of ROS in mitochondria under conditions when ROS generation by the enzyme becomes dominant over the physiological NADH generation.
3. α-KGDH in neurodegeneration
Diminished activity of α-KGDH has been linked to the mitochondrial deficiency that is crucial in the neurodegenerative process in several neurodegenerative diseases.
A characteristic change in the bioenergetic parameters in the brain of patients suffering from Parkinson's disease is a decrease in the activity of complex I of the respiratory chain (Parker et al. 1989; Schapira et al. 1989, 1990). It has been hypothesized that this could be crucial in the initiation of degeneration in the nigro-striatal dopaminergic neurons. Interestingly, the only other enzyme that has been found to be altered in the post-mortem substantia nigra samples from these patients was α-KGDH (Mizuno et al. 1990, 1994). The role of this enzyme in the pathology of the disease is underlined by the inhibitory effect of MPP+ and MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine; Joffe et al. 1998) and other structurally related isoquinoline derivatives on this enzyme (McNaught et al. 1995). α-KGDH activity is reduced also in the brain of patients suffering from Wernicke–Korsakoff syndrome (Butterworth et al. 1993).
A consistent finding was that α-KGDH activity was decreased in the brain of Alzheimer's disease patients, involving regions affected by the disease as well as regions that remained normal (Gibson et al. 1988, 2000; Butterworth & Besnard 1990; Mastrogiacomo et al. 1996). The change in the α-KGDH activity was as consistent and characteristic as the abnormal amyloid metabolism (Gibson et al. 1988, 1998). α-KGDH in fibroblasts from patients with presenilin-1 mutation was either reduced (Sheu et al. 1994) or had increased vulnerability to oxidative stress (Gibson et al. 1999). Recently, it has been reported that α-KGDH-enriched neurons are lost in the temporal cortex in Alzheimer's disease (Ko et al. 2001). The reduced enzyme activity may not be a causative event in the pathophysiology of Alzheimer's disease but the mitochondrial dysfunction due to the inhibition of α-KGDH could be a critical factor in the degeneration process (Gibson et al. 2000). The role of α-KGDH in the pathogenesis of neurodegenerative diseases is underlined by the observation that in rare cases of genetic defects of α-KGDH (Gibson et al. 2000) progressive neural degeneration was observed in patients, involving a deficient E3 subunit of α-KGDH (Kohlschutter et al. 1982).
The key role of a decreased α-KGDH activity in the events leading to cell death in the central nervous system is very clearly indicated in animal models of thiamine deficiency, in which the activity of α-KGDH is reduced in brain regions where the neurons die, whereas in regions that survive the activity of the enzyme is spared (Heroux & Butterworth 1995).
The exact role and the pathological significance of α-KGDH in the neurodegenerative processes are not clear at present, but given the crucial role in limiting the metabolism during oxidative stress as well as the ROS generation by this enzyme, it would be important to address in future studies the means by which α-KGDH can be prevented from producing excess amount of ROS and/or can be protected against oxidative damage.
Acknowledgements
The authors acknowledge the support given by OTKA, ETT, Hungarian Academy of Sciences and National Office for Research and Technology to V.A-V. Thanks are expressed to Dr Istvan Lerant for completing the figures.
Footnotes
One contribution of 18 to a Theme Issue ‘Reactive oxygen species in health and disease’.
References
- Ambrosio G, Zweier J.L, Flaherty J.T. The relationship between oxygen radical generation and impairment of myocardial energy metabolism following post-ischemic reperfusion. J. Mol. Cell Cardiol. 1991;23:1359–1374. doi: 10.1016/0022-2828(91)90183-m. 10.1016/0022-2828(91)90183-M [DOI] [PubMed] [Google Scholar]
- Ambrosio G, et al. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J. Biol. Chem. 1993;268:18 532–18 541. [PubMed] [Google Scholar]
- Andersson U, Leighton B, Young M.E, Blomstrand E, Newsholme E.A. Inactivation of aconitase and oxoglutarate dehydrogenase in skeletal muscle in vitro by superoxide anions and/or nitric oxide. Biochem. Biophys. Res. Commun. 1998;249:512–516. doi: 10.1006/bbrc.1998.9171. 10.1006/bbrc.1998.9171 [DOI] [PubMed] [Google Scholar]
- Ballou D, Palmer G, Massey V. Direct demonstration of superoxide anion production during the oxidation of reduced flavin and of its catalytic decomposition by erythrocuprein. Biochem. Biophys. Res. Commun. 1969;36:898–904. doi: 10.1016/0006-291x(69)90288-5. 10.1016/0006-291X(69)90288-5 [DOI] [PubMed] [Google Scholar]
- Bando Y, Aki K. Mechanisms of generation of oxygen radicals and reductive mobilization of ferritin iron by lipoamide dehydrogenase. J. Biochem. (Tokyo) 1991;109:450–454. doi: 10.1093/oxfordjournals.jbchem.a123402. [DOI] [PubMed] [Google Scholar]
- Beal M.F. Mitochondria, free radicals, and neurodegeneration. Curr. Opin. Neurobiol. 1996;6:661–666. doi: 10.1016/s0959-4388(96)80100-0. 10.1016/S0959-4388(96)80100-0 [DOI] [PubMed] [Google Scholar]
- Beal M.F. Experimental models of Parkinson's disease. Nat. Rev. Neurosci. 2001;2:325–334. doi: 10.1038/35072550. 10.1038/35072550 [DOI] [PubMed] [Google Scholar]
- Blasig I.E, Grune T, Schonheit K, Rohde E, Jakstadt M, Haseloff R.F, Siems W.G. 4-Hydroxynonenal, a novel indicator of lipid peroxidation for reperfusion injury of the myocardium. Am. J. Physiol. 1995;269:H14–H22. doi: 10.1152/ajpheart.1995.269.1.H14. [DOI] [PubMed] [Google Scholar]
- Bolli R, Patel B.S, Jeroudi M.O, Lai E.K, McCay P.B. Demonstration of free radical generation in ‘stunned’ myocardium of intact dogs with the use of the spin trap alpha-phenyl N-tert-butyl nitrone. J. Clin. Invest. 1988;82:476–485. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem. J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Cadenas E, Stoppani A.O. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem. J. 1976;156:435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.M, Kristal B.S, Effron M.S, Shestopalov A.I, Ullucci P.A, Sheu K.F, Blass J.P, Cooper A.J. Zn2+ inhibits alpha-ketoglutarate-stimulated mitochondrial respiration and the isolated alpha-ketoglutarate dehydrogenase complex. J. Biol. Chem. 2000;275:13 441–13 447. doi: 10.1074/jbc.275.18.13441. 10.1074/jbc.275.18.13441 [DOI] [PubMed] [Google Scholar]
- Bulos B.A, Thomas B.J, Sacktor B. Calcium inhibition of the NAD+-linked isocitrate dehydrogenase from blowfly flight muscle mitochondria. J. Biol. Chem. 1984;259:10 232–10 237. [PubMed] [Google Scholar]
- Bunik V.I. 2-Oxo acid dehydrogenase complexes in redox regulation. Eur. J. Biochem. 2003;270:1036–1042. doi: 10.1046/j.1432-1033.2003.03470.x. 10.1046/j.1432-1033.2003.03470.x [DOI] [PubMed] [Google Scholar]
- Bunik V.I, Sievers C. Inactivation of the 2-oxo acid dehydrogenase complexes upon generation of intrinsic radical species. Eur. J. Biochem. 2002;269:5004–5015. doi: 10.1046/j.1432-1033.2002.03204.x. 10.1046/j.1432-1033.2002.03204.x [DOI] [PubMed] [Google Scholar]
- Butterworth R.F, Besnard A.M. Thiamine-dependent enzyme changes in temporal cortex of patients with Alzheimer's disease. Metab. Brain Dis. 1990;5:179–184. doi: 10.1007/BF00997071. 10.1007/BF00997071 [DOI] [PubMed] [Google Scholar]
- Butterworth R.F, Kril J.J, Harper C.G. Thiamine-dependent enzyme changes in the brains of alcoholics: relationship to the Wernicke–Korsakoff syndrome. Alcohol Clin. Exp. Res. 1993;17:1084–1088. doi: 10.1111/j.1530-0277.1993.tb05668.x. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Boveris A, Ragan C.I, Stoppani A.O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch. Biochem. Biophys. 1977;180:248–257. doi: 10.1016/0003-9861(77)90035-2. 10.1016/0003-9861(77)90035-2 [DOI] [PubMed] [Google Scholar]
- Cao W, Carney J.M, Duchon A, Floyd R.A, Chevion M. Oxygen free radical involvement in ischemia and reperfusion injury to brain. Neurosci. Lett. 1988;88:233–238. doi: 10.1016/0304-3940(88)90132-2. 10.1016/0304-3940(88)90132-2 [DOI] [PubMed] [Google Scholar]
- Casley C.S, Canevari L, Land J.M, Clark J.B, Sharpe M.A. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J. Neurochem. 2002;80:91–100. doi: 10.1046/j.0022-3042.2001.00681.x. 10.1046/j.0022-3042.2001.00681.x [DOI] [PubMed] [Google Scholar]
- Chan P.C, Bielski B.H. Glyceraldehyde-3-phosphate dehydrogenase-catalyzed chain oxidation of reduced nicotinamide adenine dinucleotide by perhydroxyl radicals. J. Biol. Chem. 1980;255:874–876. [PubMed] [Google Scholar]
- Cheeseman A.J, Clark J.B. Influence of the malate–aspartate shuttle on oxidative metabolism in synaptosomes. J. Neurochem. 1988;50:1559–1565. doi: 10.1111/j.1471-4159.1988.tb03044.x. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Adam-Vizi V. Mitochondria deficient in complex I activity are depolarized by hydrogen peroxide in nerve terminals: relevance to Parkinson's disease. J. Neurochem. 2001;76:302–306. doi: 10.1046/j.1471-4159.2001.00060.x. 10.1046/j.1471-4159.2001.00060.x [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Tretter L, Adam-Vizi V. Depolarization of in situ mitochondria due to hydrogen peroxide-induced oxidative stress in nerve terminals: inhibition of alpha-ketoglutarate dehydrogenase. J. Neurochem. 1999;73:220–228. doi: 10.1046/j.1471-4159.1999.0730220.x. 10.1046/j.1471-4159.1999.0730220.x [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Tretter L, Rozsa A, Adam-Vizi V. Exacerbated responses to oxidative stress by an Na(+) load in isolated nerve terminals: the role of ATP depletion & rise of [Ca(2+)](i) J. Neurosci. 2000;20:2094–2103. doi: 10.1523/JNEUROSCI.20-06-02094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney G.J, Taegtmeyer H, Newsholme E.A. Tricarboxylic acid cycle flux and enzyme activities in the isolated working rat heart. Biochem. J. 1981;200:701–703. doi: 10.1042/bj2000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J.T, Puttfarcken P. Oxidative stress, glutamate and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Denton R.M, McCormack J.G. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett. 1980;119:1–8. doi: 10.1016/0014-5793(80)80986-0. 10.1016/0014-5793(80)80986-0 [DOI] [PubMed] [Google Scholar]
- Denton R.M, Randle P.J, Martin B.R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem. J. 1972;128:161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R.M, Richards D.A, Chin J.G. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem. J. 1978;176:899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley K.E, Votyakova T.V, Reynolds I.J. Zinc inhibition of cellular energy production: implications for mitochondria and neurodegeneration. J. Neurochem. 2003;85:563–570. doi: 10.1046/j.1471-4159.2003.01678.x. [DOI] [PubMed] [Google Scholar]
- Duchen M.R. Ca(2+)-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem. J. 1992;283:41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Nelson D, Deas J, Silver I.A. Limitation of glycolysis by hexokinase in rat brain synaptosomes during intense ion pumping. Brain Res. 1996;726:153–159. doi: 10.1016/0006-8993(96)00324-1. 10.1016/0006-8993(96)00324-1 [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur R.J, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. 10.1016/0891-5849(91)90192-6 [DOI] [PubMed] [Google Scholar]
- Frederickson C.J, Maret W, Cuajungco M.P. Zinc and excitotoxic brain injury: a new model. Neuroscientist. 2004;10:18–25. doi: 10.1177/1073858403255840. 10.1177/1073858403255840 [DOI] [PubMed] [Google Scholar]
- Gardner P.R, Fridovich I. Inactivation–reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J. Biol. Chem. 1992;267:8757–8763. [PubMed] [Google Scholar]
- Gardner P.R, Raineri I, Epstein L.B, White C.W. Superoxide radical and iron modulate aconitase activity in mammalian cells. J. Biol. Chem. 1995;270:13 399–13 405. doi: 10.1074/jbc.270.22.13399. 10.1074/jbc.270.16.9137 [DOI] [PubMed] [Google Scholar]
- Garland P.B. Some kinetic properties of pig-heart oxoglutarate dehydrogenase that provide a basis for metabolic control of the enzyme activity and also a stoicheiometric assay for coenzyme A in tissue extracts. Biochem. J. 1964;92:10C–12C. doi: 10.1042/bj0920010c. [DOI] [PubMed] [Google Scholar]
- Gazaryan I.G, Krasnikov B.F, Ashby G.A, Thorneley R.N, Kristal B.S, Brown A.M. Zinc is a potent inhibitor of thiol oxidoreductase activity and stimulates reactive oxygen species production by lipoamide dehydrogenase. J. Biol. Chem. 2002;277:10 064–10 072. doi: 10.1074/jbc.M108264200. 10.1074/jbc.M108264200 [DOI] [PubMed] [Google Scholar]
- Gibson G.E, Sheu K.F, Blass J.P, Baker A, Carlson K.C, Harding B, Perrino P. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer's disease. Arch. Neurol. 1988;45:836–840. doi: 10.1001/archneur.1988.00520320022009. [DOI] [PubMed] [Google Scholar]
- Gibson G.E, Zhang H, Sheu K.F, Bogdanovich N, Lindsay J.G, Lannfelt L, Vestling M, Cowburn R.F. Alpha-ketoglutarate dehydrogenase in Alzheimer brains bearing the APP670/671 mutation. Ann. Neurol. 1998;44:676–681. doi: 10.1002/ana.410440414. 10.1002/ana.410440414 [DOI] [PubMed] [Google Scholar]
- Gibson G.E, Park L.C, Zhang H, Sorbi S, Calingasan N.Y. Oxidative stress and a key metabolic enzyme in Alzheimer brains, cultured cells, and an animal model of chronic oxidative deficits. Ann. NY Acad. Sci. 1999;893:79–94. doi: 10.1111/j.1749-6632.1999.tb07819.x. [DOI] [PubMed] [Google Scholar]
- Gibson G.E, Park L.C, Sheu K.F, Blass J.P, Calingasan N.Y. The alpha-ketoglutarate dehydrogenase complex in neurodegeneration. Neurochem. Int. 2000;36:97–112. doi: 10.1016/s0197-0186(99)00114-x. 10.1016/S0197-0186(99)00114-X [DOI] [PubMed] [Google Scholar]
- Hall E.D, Andrus P.K, Yonkers P.A. Brain hydroxyl radical generation in acute experimental head injury. J. Neurochem. 1993;60:588–594. doi: 10.1111/j.1471-4159.1993.tb03189.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J.M. Free radicals, lipid peroxidation & cell damage. Lancet. 1984;2:1095. doi: 10.1016/s0140-6736(84)91530-7. 10.1016/S0140-6736(84)91530-7 [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J.M. The importance of free radicals and catalytic metal ions in human diseases. Mol. Aspects Med. 1985;8:89–193. doi: 10.1016/0098-2997(85)90001-9. 10.1016/0098-2997(85)90001-9 [DOI] [PubMed] [Google Scholar]
- Hansford R.G. Control of mitochondrial substrate oxidation. Curr. Top. Bioenerg. 1980;10:217–278. [Google Scholar]
- Hansford R.G, Castro F. Effects of micromolar concentrations of free calcium ions on the reduction of heart mitochondrial NAD(P) by 2-oxoglutarate. Biochem. J. 1981;198:525–533. doi: 10.1042/bj1980525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M.E, Hensley K, Butterfield D.A, Leedle R.A, Carney J.M. Direct evidence of oxidative injury produced by the Alzheimer's beta-amyloid peptide (1-40) in cultured hippocampal neurons. Exp. Neurol. 1995;131:193–202. doi: 10.1016/0014-4886(95)90041-1. 10.1016/0014-4886(95)90041-1 [DOI] [PubMed] [Google Scholar]
- Heroux M, Butterworth R.F. Regional alterations of thiamine phosphate esters and of thiamine diphosphate-dependent enzymes in relation to function in experimental Wernicke's encephalopathy. Neurochem. Res. 1995;20:87–93. doi: 10.1007/BF00995157. 10.1007/BF00995157 [DOI] [PubMed] [Google Scholar]
- Herrero A, Barja G. Localization of the site of oxygen radical generation inside the complex I of heart and nonsynaptic brain mammalian mitochondria. J. Bioenerg. Biomembr. 2000;32:609–615. doi: 10.1023/a:1005626712319. 10.1023/A:1005626712319 [DOI] [PubMed] [Google Scholar]
- Huennekens F.M, Basford R.E, Gabrio B.W. An oxidase for reduced diphosphopyridine nucleotide. J. Biol. Chem. 1955;213:951–967. [PubMed] [Google Scholar]
- Humphries K.M, Szweda L.I. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37:15 835–15 841. doi: 10.1021/bi981512h. 10.1021/bi981512h [DOI] [PubMed] [Google Scholar]
- Humphries K.M, Yoo Y, Szweda L.I. Inhibition of NADH-linked mitochondrial respiration by 4-hydroxy-2-nonenal. Biochemistry. 1998;37:552–557. doi: 10.1021/bi971958i. 10.1021/bi971958i [DOI] [PubMed] [Google Scholar]
- Hyslop P.A, Zhang Z, Pearson D.V, Phebus L.A. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2 in vitro. Brain Res. 1995;671:181–186. doi: 10.1016/0006-8993(94)01291-o. 10.1016/0006-8993(94)01291-O [DOI] [PubMed] [Google Scholar]
- Joffe G.T, Parks J.K, Parker W.D., Jr Secondary inhibition of 2-ketoglutarate dehydrogenase complex by MPTP. Neuroreport. 1998;9:2781–2783. doi: 10.1097/00001756-199808240-00018. [DOI] [PubMed] [Google Scholar]
- Kakinuma K, Fukuhara Y, Kaneda M. The respiratory burst oxidase of neutrophils. Separation of an FAD enzyme and its characterization. J. Biol. Chem. 1987;262:12 316–12 322. [PubMed] [Google Scholar]
- Ko L.W, Sheu K.F, Thaler H.T, Markesbery W.R, Blass J.P. Selective loss of KGDHC-enriched neurons in Alzheimer temporal cortex: does mitochondrial variation contribute to selective vulnerability? J. Mol. Neurosci. 2001;17:361–369. doi: 10.1385/JMN:17:3:361. 10.1385/JMN:17:3:361 [DOI] [PubMed] [Google Scholar]
- Koh J.Y, Suh S.W, Gwag B.J, He Y.Y, Hsu C.Y, Choi D.W. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Kohlschutter A, Behbehani A, Langenbeck U, Albani M, Heidemann P, Hoffmann G, Kleineke J, Lehnert W, Wendel U. A familial progressive neurodegenerative disease with 2-oxoglutaric aciduria. Eur. J. Pediatr. 1982;138:32–37. doi: 10.1007/BF00442325. [DOI] [PubMed] [Google Scholar]
- Koike M, Koike K. Structure, assembly and function of mammalian alpha-keto acid dehydrogenase complexes. Adv. Biophys. 1976;9:182–227. [PubMed] [Google Scholar]
- Koike K, Hamada M, Tanaka N, Otsuka K.I, Ogasahara K, Koike M. Properties and subunit composition of the pig heart 2-oxoglutarate dehydrogenase. J. Biol. Chem. 1974;249:3836–3842. [PubMed] [Google Scholar]
- Korshunov S.S, Skulachev V.P, Starkov A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. 10.1016/S0014-5793(97)01159-9 [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Culcasi M, Gaven F, Pietri S, Bockaert J. Nitric oxide, superoxide and peroxynitrite: putative mediators of NMDA-induced cell death in cerebellar granule cells. Neuropharmacology. 1993;32:1259–1266. doi: 10.1016/0028-3908(93)90020-4. 10.1016/0028-3908(93)90020-4 [DOI] [PubMed] [Google Scholar]
- Lai J.C, Cooper A.J. Brain alpha-ketoglutarate dehydrogenase complex: kinetic properties, regional distribution and effects of inhibitors. J. Neurochem. 1986;47:1376–1386. doi: 10.1111/j.1471-4159.1986.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Lai J.C, Walsh J.M, Dennis S.C, Clark J.B. Synaptic and non-synaptic mitochondria from rat brain: isolation and characterization. J. Neurochem. 1977;28:625–631. doi: 10.1111/j.1471-4159.1977.tb10434.x. [DOI] [PubMed] [Google Scholar]
- Leong S.F, Clark J.B. Regional enzyme development in rat brain. Enzymes of energy metabolism. Biochem. J. 1984;218:139–145. doi: 10.1042/bj2180139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong S.F, Lai J.C, Lim L, Clark J.B. Energy-metabolizing enzymes in brain regions of adult and aging rats. J. Neurochem. 1981;37:1548–1556. doi: 10.1111/j.1471-4159.1981.tb06326.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. 10.1046/j.0022-3042.2002.00744.x [DOI] [PubMed] [Google Scholar]
- Loschen G, Azzi A, Flohe L. Mitochondrial H2O2 formation: relationship with energy conservation. FEBS Lett. 1973;33:84–87. doi: 10.1016/0014-5793(73)80165-6. 10.1016/0014-5793(73)80165-6 [DOI] [PubMed] [Google Scholar]
- Loschen G, Azzi A, Richter C, Flohe L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. 10.1016/0014-5793(74)80281-4 [DOI] [PubMed] [Google Scholar]
- Lucas D.T, Szweda L.I. Declines in mitochondrial respiration during cardiac reperfusion: age-dependent inactivation of alpha-ketoglutarate dehydrogenase. Proc. Natl Acad. Sci. USA. 1999;96:6689–6693. doi: 10.1073/pnas.96.12.6689. 10.1073/pnas.96.12.6689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark R.J, Lovell M.A, Markesbery W.R, Uchida K, Mattson M.P. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J. Neurochem. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- Massey V. Activation of molecular oxygen by flavins and flavoproteins. J. Biol. Chem. 1994;269:22 459–22 462. [PubMed] [Google Scholar]
- Massey V, Strickland S, Mayhew S.G, Howell L.G, Engel P.C, Matthews R.G, Schuman M, Sullivan P.A. The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem. Biophys. Res. Commun. 1969;36:891–897. doi: 10.1016/0006-291x(69)90287-3. 10.1016/0006-291X(69)90287-3 [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo F, Bergeron C, Kish S.J. Brain alpha-ketoglutarate dehydrogenase complex activity in Alzheimer's disease. J. Neurochem. 1993;61:2007–2014. doi: 10.1111/j.1471-4159.1993.tb07436.x. [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo F, LaMarche J, Dozic S, Lindsay G, Bettendorff L, Robitaille Y, Schut L, Kish S.J. Immunoreactive levels of alpha-ketoglutarate dehydrogenase subunits in Friedreich's ataxia and spinocerebellar ataxia type 1. Neurodegeneration. 1996;5:27–33. doi: 10.1006/neur.1996.0004. 10.1006/neur.1996.0004 [DOI] [PubMed] [Google Scholar]
- McCormack J.G, Denton R.M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem. J. 1979;180:533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J.G, Longo E.A, Corkey B.E. Glucose-induced activation of pyruvate dehydrogenase in isolated rat pancreatic islets. Biochem. J. 1990;267:527–530. doi: 10.1042/bj2670527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaught K.S, Altomare C, Cellamare S, Carotti A, Thull U, Carrupt P.A, Testa B, Jenner P, Marsden C.D. Inhibition of alpha-ketoglutarate dehydrogenase by isoquinoline derivatives structurally related to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Neuroreport. 1995;6:1105–1108. doi: 10.1097/00001756-199505300-00008. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Suzuki K, Ohta S. Postmortem changes in mitochondrial respiratory enzymes in brain and a preliminary observation in Parkinson's disease. J. Neurol. Sci. 1990;96:49–57. doi: 10.1016/0022-510x(90)90056-s. 10.1016/0022-510X(90)90056-S [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Matuda S, Yoshino H, Mori H, Hattori N, Ikebe S. An immunohistochemical study on alpha-ketoglutarate dehydrogenase complex in Parkinson's disease. Ann. Neurol. 1994;35:204–210. doi: 10.1002/ana.410350212. 10.1002/ana.410350212 [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Yoshino H, Ikebe S, Hattori N, Kobayashi T, Shimoda-Matsubayashi S, Matsumine H, Kondo T. Mitochondrial dysfunction in Parkinson's disease. Ann. Neurol. 1998;44:S99–S109. doi: 10.1002/ana.410440715. 10.1002/ana.410440116 [DOI] [PubMed] [Google Scholar]
- Moreno-Sanchez R, Hogue B.A, Hansford R.G. Influence of NAD-linked dehydrogenase activity on flux through oxidative phosphorylation. Biochem. J. 1990;268:421–428. doi: 10.1042/bj2680421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nulton-Persson A.C, Szweda L.I. Modulation of mitochondrial function by hydrogen peroxide. J. Biol. Chem. 2001;276:23 357–23 361. doi: 10.1074/jbc.M100320200. 10.1074/jbc.M100320200 [DOI] [PubMed] [Google Scholar]
- Olanow C.W. A radical hypothesis for neurodegeneration. Trends Neurosci. 1993;16:439–444. doi: 10.1016/0166-2236(93)90070-3. 10.1016/0166-2236(93)90070-3 [DOI] [PubMed] [Google Scholar]
- Park L.C, Zhang H, Sheu K.F, Calingasan N.Y, Kristal B.S, Lindsay J.G, Gibson G.E. Metabolic impairment induces oxidative stress, compromises inflammatory responses and inactivates a key mitochondrial enzyme in microglia. J. Neurochem. 1999;72:1948–1958. doi: 10.1046/j.1471-4159.1999.0721948.x. 10.1046/j.1471-4159.1999.0721948.x [DOI] [PubMed] [Google Scholar]
- Parker W.D, Jr, Boyson S.J, Parks J.K. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann. Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. 10.1002/ana.410260606 [DOI] [PubMed] [Google Scholar]
- Patel M, Day B.J, Crapo J.D, Fridovich I, McNamara J.O. Requirement for superoxide in excitotoxic cell death. Neuron. 1996;16:345–355. doi: 10.1016/s0896-6273(00)80052-5. 10.1016/S0896-6273(00)80052-5 [DOI] [PubMed] [Google Scholar]
- Pettit F.H, Roche T.E, Reed L.J. Function of calcium ions in pyruvate dehydrogenase phosphatase activity. Biochem. Biophys. Res. Commun. 1972;49:563–571. doi: 10.1016/0006-291x(72)90448-2. 10.1016/0006-291X(72)90448-2 [DOI] [PubMed] [Google Scholar]
- Pralong W.F, Hunyady L, Varnai P, Wollheim C.B, Spat A. Pyridine nucleotide redox state parallels production of aldosterone in potassium-stimulated adrenal glomerulosa cells. Proc. Natl Acad. Sci. USA. 1992;89:132–136. doi: 10.1073/pnas.89.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds I.J, Hastings T.G. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J. Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek H.A, Humphries K.M, Szweda P.A, Szweda L.I. Selective inactivation of redox-sensitive mitochondrial enzymes during cardiac reperfusion. Arch. Biochem. Biophys. 2002;406:222–228. doi: 10.1016/s0003-9861(02)00446-0. 10.1016/S0003-9861(02)00446-0 [DOI] [PubMed] [Google Scholar]
- Scanlon J.M, Reynolds I.J. Effects of oxidants and glutamate receptor activation on mitochondrial membrane potential in rat forebrain neurons. J. Neurochem. 1998;71:2392–2400. doi: 10.1046/j.1471-4159.1998.71062392.x. [DOI] [PubMed] [Google Scholar]
- Schapira A.H. Mitochondrial involvement in Parkinson's disease, Huntington's disease, hereditary spastic paraplegia and Friedreich's ataxia. Biochim. Biophys. Acta. 1999;1410:159–170. doi: 10.1016/s0005-2728(98)00164-9. [DOI] [PubMed] [Google Scholar]
- Schapira A.H, Cooper J.M, Dexter D, Jenner P, Clark J.B, Marsden C.D. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. 10.1016/S0140-6736(89)92366-0 [DOI] [PubMed] [Google Scholar]
- Schapira A.H, Cooper J.M, Dexter D, Clark J.B, Jenner P, Marsden C.D. Mitochondrial complex I deficiency in Parkinson's disease. J. Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schmidley J.W. Free radicals in central nervous system ischemia. Stroke. 1990;21:1086–1090. doi: 10.1161/01.str.21.7.1086. [DOI] [PubMed] [Google Scholar]
- Sensi S.L, Yin H.Z, Carriedo S.G, Rao S.S, Weiss J.H. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc. Natl Acad. Sci. USA. 1999;96:2414–2419. doi: 10.1073/pnas.96.5.2414. 10.1073/pnas.96.5.2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank R.P, Campbell G.L. Alpha-ketoglutarate and malate uptake and metabolism by synaptosomes: further evidence for an astrocyte-to-neuron metabolic shuttle. J. Neurochem. 1984;42:1153–1161. doi: 10.1111/j.1471-4159.1984.tb12724.x. [DOI] [PubMed] [Google Scholar]
- Sheu K.F, Kim Y.T, Blass J.P, Weksler M.E. An immunochemical study of the pyruvate dehydrogenase deficit in Alzheimer's disease brain. Ann. Neurol. 1985;17:444–449. doi: 10.1002/ana.410170505. 10.1002/ana.410170505 [DOI] [PubMed] [Google Scholar]
- Sheu K.F, Cooper A.J, Koike K, Koike M, Lindsay J.G, Blass J.P. Abnormality of the alpha-ketoglutarate dehydrogenase complex in fibroblasts from familial Alzheimer's disease. Ann. Neurol. 1994;35:312–318. doi: 10.1002/ana.410350311. 10.1002/ana.410350311 [DOI] [PubMed] [Google Scholar]
- Siesjo B.K, Zhao Q, Pahlmark K, Siesjo P, Katsura K, Folbergrova J. Glutamate, calcium and free radicals as mediators of ischemic brain damage. Ann. Thorac. Surg. 1995;59:1316–1320. doi: 10.1016/0003-4975(95)00077-x. [DOI] [PubMed] [Google Scholar]
- Sipos I, Tretter L, Adam-Vizi V. Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. J. Neurochem. 2003a;84:112–118. doi: 10.1046/j.1471-4159.2003.01513.x. 10.1046/j.1471-4159.2003.01513.x [DOI] [PubMed] [Google Scholar]
- Sipos I, Tretter L, Adam-Vizi V. The production of reactive oxygen species in intact isolated nerve terminals is independent of the mitochondrial membrane potential. Neurochem. Res. 2003b;28:1575–1581. doi: 10.1023/a:1025634728227. 10.1023/A:1025634728227 [DOI] [PubMed] [Google Scholar]
- Smith C.M, Bryla J, Williamson J.R. Regulation of mitochondrial alpha-ketoglutarate metabolism by product inhibition at alpha-ketoglutarate dehydrogenase. J. Biol. Chem. 1974;249:1497–1505. [PubMed] [Google Scholar]
- Starkov A.A, Fiskum G. Myxothiazol induces H(2)O(2) production from mitochondrial respiratory chain. Biochem. Biophys. Res. Commun. 2001;281:645–650. doi: 10.1006/bbrc.2001.4409. 10.1006/bbrc.2001.4409 [DOI] [PubMed] [Google Scholar]
- Starkov A.A, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J. Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- Starkov A.A, Fiskum G, Chinopoulos C, Lorenzo B.J, Browne S.E, Patel M.S, Beal M.F. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J. Neurosci. 2004;24:7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. 10.1523/JNEUROSCI.1899-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshige K, Minakami S. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem. J. 1979;180:129–135. doi: 10.1042/bj1800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwel D, Bothmer J, Wolf E, Meng F, Jolles J. Affected enzyme activities in Alzheimer's disease are sensitive to antemortem hypoxia. J. Neurol. Sci. 1998;161:47–56. doi: 10.1016/s0022-510x(98)00240-8. 10.1016/S0022-510X(98)00240-8 [DOI] [PubMed] [Google Scholar]
- Tonder N, Johansen F.F, Frederickson C.J, Zimmer J, Diemer N.H. Possible role of zinc in the selective degeneration of dentate hilar neurons after cerebral ischemia in the adult rat. Neurosci. Lett. 1990;109:247–252. doi: 10.1016/0304-3940(90)90002-q. 10.1016/0304-3940(90)90002-Q [DOI] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J. Neurosci. 2000;20:8972–8979. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J. Neurosci. 2004;24:7771–7778. doi: 10.1523/JNEUROSCI.1842-04.2004. 10.1523/JNEUROSCI.1842-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L, Chinopoulos C, Adam-Vizi V. Enhanced depolarization-evoked calcium signal and reduced [ATP]/[ADP] ratio are unrelated events induced by oxidative stress in synaptosomes. J. Neurochem. 1997;69:2529–2537. doi: 10.1046/j.1471-4159.1997.69062529.x. [DOI] [PubMed] [Google Scholar]
- Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. 10.1113/jphysiol.2003.049478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens J.F, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votyakova T.V, Reynolds I.J. DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. 10.1046/j.1471-4159.2001.00548.x [DOI] [PubMed] [Google Scholar]
- Wang G.J, Thayer S.A. Sequestration of glutamate-induced Ca2+ loads by mitochondria in cultured rat hippocampal neurons. J. Neurophysiol. 1996;76:1611–1621. doi: 10.1152/jn.1996.76.3.1611. [DOI] [PubMed] [Google Scholar]
- Weiss J.H, Sensi S.L, Koh J.Y. Zn2+: a novel ionic mediator of neural injury in brain disease. Trends Pharmacol. Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. 10.1016/S0165-6147(00)01541-8 [DOI] [PubMed] [Google Scholar]
- White R.J, Reynolds I.J. Mitochondrial depolarization in glutamate-stimulated neurons: an early signal specific to excitotoxin exposure. J. Neurosci. 1996;16:5688–5697. doi: 10.1523/JNEUROSCI.16-18-05688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby J, Craig F.E, Harvey S.A, Clark J.B. 2-Oxoglutarate: oxidation and role as a potential precursor of cytosolic acetyl-CoA for the synthesis of acetylcholine in rat brain synaptosomes. J. Neurochem. 1989;52:896–901. doi: 10.1111/j.1471-4159.1989.tb02539.x. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Nelson D, Daikhin Y, Erecinska M. Tricarboxylic acid cycle in rat brain synaptosomes. Fluxes and interactions with aspartate aminotransferase and malate/aspartate shuttle. J. Biol. Chem. 1994;269:27 414–27 420. [PubMed] [Google Scholar]
- Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies K.J. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J. Biol. Chem. 1990;265:16 330–16 336. [PubMed] [Google Scholar]
- Zhang L, Yu L, Yu C.A. Generation of superoxide anion by succinate-cytochrome c reductase from bovine heart mitochondria. J. Biol. Chem. 1998;273:33 972–33 976. doi: 10.1074/jbc.273.51.33972. 10.1074/jbc.273.51.33972 [DOI] [PubMed] [Google Scholar]