Abstract
Skeletal muscle has been shown to generate a complex set of reactive oxygen and nitrogen species (ROS) both at rest and during contractile activity. The primary ROS generated are superoxide and nitric oxide and the pattern and magnitude of their generation is influenced by the nature of the contractile activity. It is increasingly clear that the ROS generated by skeletal muscle play an important role in influencing redox-regulated processes that control, at least some of, the adaptive responses to contractile activity. These processes are also recognized to be modified during ageing and in some disease states, providing the potential that interventions affecting ROS activity may influence muscle function or viability in these situations.
Keywords: contractile activity, myotube, nitric oxide, superoxide
1. Skeletal muscle adapts rapidly to changes in stretch or contractile activity
Numerous studies indicate that skeletal muscle adapts rapidly to the type of work it is required to perform and/or the pattern of contractile activity undertaken such that specific responses in composition occur following changes in the pattern of contractile activity or external forces such as passive stretching. These adaptations lead to an improved ability of the tissue to deal with the increased stress. There are many comprehensive descriptions of the pattern of these responses and increasing amounts of published information on the signalling pathways involved (Widegren et al. 1998; Nader & Esser 2001; Hawley 2002). Manipulation or correction of aberrant adaptive responses requires a comprehensive understanding of the molecular and biochemical processes involved, but currently little is known about the factors that stimulate adaptive responses to stretch or contractile activity (Goldspink 1994; Nader & Esser 2001; Hawley 2002).
2. Skeletal muscle generates a number of reactive oxygen species that are increased during contractile activity
Many studies have demonstrated an increase in end-point indicators of the reactions of reactive oxygen species (ROS) in tissues during and following exercise (e.g. Dillard et al. 1978; Davies et al. 1982; Jackson et al. 1985). This increase in ROS activity appears to be in major part due to generation by contracting skeletal muscle. The primary ROS generated by skeletal muscle during activity are NO and superoxide and the superoxide dismutates rapidly to form hydrogen peroxide (McArdle & Jackson 2000). There are a number of potential sites for NO and superoxide generation in skeletal muscle (Jackson 2000). This tissue also has a well-developed system to regulate these ROS and prevent potentially deleterious effects. These protective systems include both mitochondrial and cytosolic isoforms of superoxide dismutase (MnSOD and CuZnSOD, respectively), catalase and glutathione peroxidase enzymes and a number of direct scavengers of ROS including glutathione, vitamin E and ascorbate. In general, slow twitch, mitochondria-rich (type I) fibres have an increased content of protective systems in comparison with fast (type II) fibres.
A clear understanding of the mechanisms of ROS generation in contracting skeletal muscle has not been obtained, in part due to a lack of suitable analytical techniques. We have developed complimentary approaches to this problem: (i) microdialysis techniques to study ROS in muscle interstitial fluid (McArdle et al. 2001; Pattwell et al. 2001) and (ii) the release of ROS from contracting myotubes in culture (McArdle et al. 2001; Pattwell et al. 2004), that have allowed us to obtain a greater understanding of the release and generation of specific ROS by muscle.
(a) Nitric oxide is generated by skeletal muscle during activity
NO is generated continuously by skeletal muscle, a production that is increased by contractions (Balon & Nadler 1994). Skeletal muscle normally expresses the neuronal (type I or nNOS) and the endothelial (type III or eNOS) isoforms of nitric oxide synthase (NOS). nNOS is strongly expressed in fast-twitch muscle fibres and localized to the muscle sacrolemma, where it is associated with the dystrophin–glycoprotein complex (DGC). eNOS is localized to the muscle mitochondria (Kobzik et al. 1995). iNOS (type II) is also expressed in skeletal muscle in some inflammatory conditions, but it does not play a significant role in normal muscle (Stamler & Meissner 2001). Release of NO was originally demonstrated from isolated muscles in vitro (Balon & Nadler 1994), although the cellular source of the NO released was unclear. Our recent analysis of myotubes in culture has confirmed that skeletal muscle cells per se release increased amounts of NO during contractile activity, (Pattwell et al. 2004), a release that was greatly reduced by the NOS inhibitor, l-NAME. nNOS appears to be the prime source of the NO released from skeletal muscle (Hirschfield et al. 2000). Passive stretching of muscle has also been shown to increase NO release from rat skeletal muscle in vitro (Tidball et al. 1998), and nNOS expression is increased by repeated exposure of muscle to contractile activity or passive stretching (Roberts et al. 1999; Tidball et al. 1998).
(b) Superoxide anion is released by skeletal muscle during activity
Studies with isolated strips of diaphragm (Reid et al. 1992) and in vivo microdialysis studies (McArdle et al. 2001) indicate that increased amounts of superoxide anion are released into the muscle extracellular fluid during contractile activity. Complementary studies of myotubes in culture indicated that a major source of this superoxide is skeletal muscle cells (McArdle et al. 2001). Although standard texts cite mitochondria as the major site of superoxide generation (Halliwell & Gutteridge 1989), a number of observations suggest that this extracellular superoxide does not originate from mitochondrial generation. Superoxide is charged and reactive and does not readily cross membranes and although myotubes in culture contain few mitochondria, these cells release relatively large amounts of superoxide during contractile activity (McArdle et al. 2001). In vivo microdialysis studies with knockout mice also support this. Mice with reduced mitochondrial MnSOD activity (Sod2+/−) have evidence of increased mitochondrial superoxide (Van Remmen et al. 1999), but baseline levels of extracellular superoxide and the increase during contractile activity were unchanged in the Sod2+/− compared with wild type mice (McArdle et al. 2004c). Potential alternative sources for extracellular superoxide release include plasma membrane oxidoreductases and our inhibitor studies indicate that superoxide release from stimulated myotubes was reduced by treatment with diphenylene iodonium, a non-specific inhibitor of NAD(P)H oxidases and other flavoproteins (Pattwell et al. 2004). NAD(P)H oxidase is expressed in diaphragm muscle (Javesghani et al. 2002) and this, and other, muscle plasma membrane oxidoreductases may contribute to extracellular superoxide (de Grey 2000; Morre et al. 2000).
Chronic contractile activity also appears to influence the ability of muscle to detoxify superoxide with increases in skeletal muscle total SOD (McArdle et al. 2001), MnSOD (Ji 1993) and extracellular SOD (Fukai et al. 2000) activities reported following single or repeated periods of contractile activity. Our preliminary microdialysis studies also indicate that mouse skeletal muscle releases hydrogen peroxide into the interstitial fluid during contractile activity (data not shown in detail). Although a portion of this hydrogen peroxide is likely to derive from the superoxide released into the interstitial fluid, our data obtained from animals with reduced muscle MnSOD activity (Sod2+/−) indicate that the majority of the increase in extracellular hydrogen peroxide during contractile activity may originate in the muscle mitochondria and diffuse across intracellular and plasma membranes (McArdle et al. 2004c).
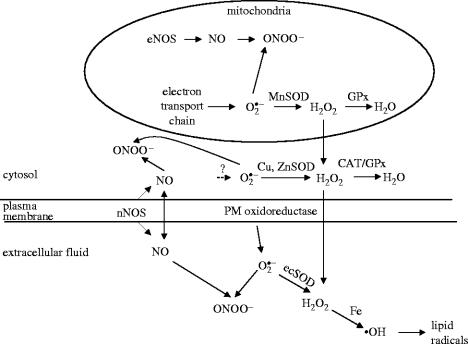
As a result of these and other data we have proposed the scheme for skeletal muscle ROS generation shown in figure 1. Current data indicate that the plasma membrane superoxide and NO generating systems can be activated by either mechanical distention or contractile activity. In contrast, a relatively large rise in hydrogen peroxide release is only seen following an increase in muscle mitochondrial activity such as occurs with aerobic contractile activity.
Figure 1.
Schematic diagram depicting the different sites and interactions of ROS and NO generated by skeletal muscle cells. GPx, glutathione peroxidase. Modified from Pattwell & Jackson (2004).
3. Functions of ROS in skeletal muscle
Much previous work has examined the possibility that ROS are mediators of contraction-induced damage to skeletal muscle (see Reznick et al. 1998 for reviews). It is clear that ROS can exert such effects in situations where antioxidant defences are compromised (e.g. Jackson et al. 1983) or where ROS production is grossly excessive (e.g. McArdle et al. 1999), but evidence that ROS have these effects under physiological conditions in vivo is sparse. In contrast, it has recently become clear that ROS interact with multiple cell signalling and regulatory pathways to modulate changes in gene expression (see Dröge 2001; Haddad 2002; Jackson et al. 2002 for reviews).
(a) Roles of NO in skeletal muscle
NO within, and released from, skeletal muscle cells has a number of general functions (Grozdanovic 2001). The functions of many cellular proteins are modified by NO, including soluble guanyl cyclase and mitochondrial respiratory chain complexes. In skeletal muscle, NO has been implicated in the regulation of contractility, mitochondrial oxygen metabolism, glucose homeostasis and blood flow (Stamler & Meissner 2001). Of particular interest is the role of NO in signalling adaptive responses to contractile activity or stretch. Tidball and co-workers demonstrated that NOS activity mediated sarcomere addition during remobilization of muscle (Koh & Tidball 1999) and that NOS activity mediated increased expression of the structural proteins talin and vinculin during cyclic stretching of skeletal muscle (Tidball et al. 1999). In addition both NO and peroxynitrite have effects on redox sensitive regulating elements in signalling changes in gene expression and can induce changes in gene expression in many systems (see Dröge 2001; Jackson et al. 2002 for reviews).
(b) Roles of superoxide/hydrogen peroxide in skeletal muscle
There is relatively little information on the roles of superoxide in regulation of skeletal muscle, but increasing evidence that the dismutation product, hydrogen peroxide, can play important roles. These species are reported to modulate skeletal muscle force generation and fatigue (see Reid 2001 for a review) although these are not universal findings. Additionally hydrogen peroxide may exert effects on vasodilation (Miura et al. 2003). There has been considerable interest in the potential roles of superoxide/hydrogen peroxide in signalling adaptive responses in many cell types (see Jackson et al. 2002 for a review) and some changes in cell signalling following contractile activity in skeletal muscle are reported to be mediated by ROS (Wretman et al. 2001). We have obtained a number of pieces of evidence that contraction-induced ROS modulate at least some of the adaptive and stress responses that occur in skeletal muscle following contractile activity. A single period of contractile activity in mouse muscle was found to increase muscle SOD and catalase activity together with HSP60 and HSP70 content (McArdle et al. 2001), changes which were replicated in human muscle (Khassaf et al. 2001). Pre-supplementation with vitamin C reduced these responses, supporting the possibility that they are regulated by ROS (Khassaf et al. 2003).
The possibility that ROS can directly modulate stress responses to contraction in skeletal muscle has been examined by using Clontech cDNA expression arrays to determine whether hydrogen peroxide treatment could induce changes in stress gene expression in skeletal muscle in culture and comparison of these changes with those seen in skeletal muscle in vivo at the same time points following a period of non-damaging contractile activity known to induce a substantial generation of oxidants (McArdle et al. 2001). Treatment of myotubes with hydrogen peroxide induced a significant change in the expression of 19 of the 140 genes on the array at 4 h post-exposure. These mainly showed a reduction in expression. Following contractile activity in skeletal muscle, 23 mRNA species showed a significant change in expression at 4 h. A comparison of the pattern of changes in the mRNA from the two models identified six genes showing the same pattern with both stresses (McArdle et al. 2004a). It can be argued that changes in the expression of these six genes may represent part of an oxidant-mediated response to contractile activity. These are likely to be only a small portion of the genes whose expression is modulated by ROS because of the limited number of genes on these arrays. The six genes identified all respond to hydrogen peroxide in muscle cells and are modulated in a similar manner by contractile activity, but only one of these mRNAs showed upregulation following contractile activity: haem oxygenase-1. The validity of these array data is supported by data showing that haem oxygenase-1 protein is increased in skeletal muscle following contractile activity (Pilegaard et al. 2000) and in myotubes following hydrogen peroxide treatment (McArdle et al. 2004a).
One mechanism by which cells respond to ROS is through activation of certain transcription factors (Jackson et al. 2002), and supportive evidence for a role of superoxide in mediating adaptive responses to contractions also comes from recent studies of transcription factor activation. Data indicate that NFκB binding to DNA was increased following contractile activity (Ji et al. 2004).
Overall, therefore, it now appears that both NO and superoxide play roles in muscle regulation and adaptations to activity although the overall extent and nature of these roles remains to be fully evaluated.
4. Interactions of NO and superoxide
NO reacts with superoxide to generate peroxynitrite (ONOO−), a reaction that is three times more efficient than SOD in scavenging superoxide. Hence, peroxynitrite formation is preferred, where both radical species are present (Halliwell & Gutteridge 1989). The presence of one of these species can, therefore, affect the ‘bioavailability’ of the other: NO can reduce superoxide toxicity and conversely superoxide can decrease NO availability and inhibit effects such as vasodilation. Such effects have been studied in endothelial cells (Cosentino & Luscher 1999; Hwang et al. 2003), but not in skeletal muscle. There is also evidence for a co-ordinated regulation of pathways influencing superoxide and NO levels. Exercise increases muscle NO and superoxide generation and leads to increases in muscle nNOS and intracellular and extracellular SOD activities (Roberts et al. 1999; McArdle et al. 2001; Fukai et al. 2000). NO or superoxide also appear to influence the regulatory pathways for the other compound: an increase in hydrogen peroxide content is reported to stimulate NOS expression in endothelial cells (Drummond et al. 2000), while elevated levels of NO decrease extracellular SOD activity in smooth muscle cells (Strålin et al. 2003). We tested the hypothesis that NO and superoxide react in the muscle extracellular space following increased release from contracting myotubes by measuring superoxide release from wild type cells when NOS was inhibited by l-NAME treatment. NOS inhibition increased the superoxide anions detected (Pattwell et al. 2004), demonstrating indirectly that peroxynitrite was formed, where both species were present. We have also examined the possibility that peroxynitrite is formed following contractile activity in myotubes by western blotting analysis of nitrotyrosine residues in muscle proteins. Nitrotyrosines were increased following a single period of contractile activity (data not shown in detail). We conclude that peroxynitrite may be formed from NO and superoxide generated by muscle cells, reducing the effective bioavailability of both. This appears to provide a system whereby relatively small changes in generation of one of the primary ROS (i.e. NO or superoxide) leads to sensitive changes in several other substances that potentially influence cellular responses. Thus, for example, a decrease in NO generation will lead to a decrease in peroxynitrite formation and an increase in superoxide and hydrogen peroxide content. In other cell types an imbalance of NO and superoxide has been recognized to cause functional changes (Cosentino et al. 2001), but this possibility does not appear to have been examined in skeletal muscle.
5. NOS enzymes are a potential source of both NO and superoxide
NOS enzymes contain both reductase and oxygenase domains. Formation of NO involves an electron transfer from NADPH bound at the reductase domain to the haem centre of the oxygenase domain. Calmodulin binding appears to facilitate this transfer. NOS enzymes have tetrahydrobiopterin as a cofactor that mediates coupling of oxygen reduction to haem catalysed l-arginine oxidation to form NO and citrulline. In recent years in has become apparent that when NOS enzymes lack substrate (arginine), cofactor (tetrahydrobiopterin) or specific protein–protein interactions (Xia et al. 1996; Cosentino et al. 1998; Bender et al. 1999; Pou et al. 1999; Song et al. 2002; Ou et al. 2003), the oxygen reduction and arginine oxidation become ‘uncoupled’ leading to generation of superoxide by the enzymes. In endothelial cells this uncoupling leads to superoxide-mediated endothelial dysfunction (Cosentino & Lüscher 1999; Cosentino et al. 2001). The potential for uncoupling appears to apply to all three NOS isoforms but, again, this does not appear to have been examined in skeletal muscle.
6. Superoxide/nitric oxide interactions in muscle degeneration
Aberrant production or regulation of NO and/or superoxide have been claimed to play a role in some degenerative disorders of muscle (see Reznick et al. 1998 for a review). Of potential importance, but poorly understood, are roles in the muscular dystrophies and in muscle dysfunction associated with ageing.
(a) Muscular dystrophies
The muscular dystrophies are a group of inherited disorders of muscle characterized by significant muscle degeneration and weakness (Partridge 1993). Dystrophin, the defective protein in the most common form, Duchenne muscular dystrophy (DMD), is a component of a complex set of proteins and glycoproteins associated with the cell membrane, several of which may cause other forms of muscular dystrophy if defective or absent (Durbeej & Campbell 2002). The functions of many components of the DGC complex are unclear. The skeletal muscle isoform of nNOS, termed nNOSμ (or muNOS), is associated with the complex (Brenman et al. 1995). Loss of dystrophin protein (as occurs in DMD patients or in the mdx mouse model) leads to complete loss of the DGC and nNOS from the sarcolemma (Durbeej & Campbell 2002). nNOS is also lost from the sarcolemma in mice deficient in other components of the DGC (Crosbie et al. 2002). Loss of nNOS from the sarcolemma leads to redistribution of nNOS to the cytosol. Estimates of the residual nNOS present in the cytosol in dystrophin-deficient muscle vary greatly from an overall decrease to a 75% increase (Brennan et al. 1995; Chang et al. 1996). There is evidence that reversal of this loss of nNOS by overexpression of the protein in mdx mice partially ameliorates the degeneration in this model (Wehling et al. 2001), providing strong support that changes in redox regulation may be important in this disorder.
(b) ROS generation in muscle of ageing mice
There is evidence that abnormalities in muscle NO/superoxide interactions play a role in ageing-related muscle dysfunction. Loss of muscle strength and muscle wasting are characteristic of ageing and are caused by both a loss of muscle fibres and atrophy of the remaining fibres (Porter et al. 1995). All tissues of aged organisms contain products of oxidative damage to biomolecules such as phospholipid, DNA and proteins (Nohl 1993; Schoneich 1999). Aged mitochondria contain significant amounts of oxidative damage associated with a marked increase in the number of rearrangements of mitochondrial DNA (Melov et al. 1995). Sohal and co-workers (Sohal et al. 1994; Lass et al. 1998) suggest that these changes are due to an increase in mitochondrial superoxide and hydrogen peroxide production with increasing age although there is little direct evidence to support this hypothesis. Lass et al. (1998) examined the superoxide generation from sub-mitochondrial particles from skeletal muscle and reported an increased release in aged animals while Bejma & Ji (1999) reported that homogenates of skeletal muscle from old animals induced greater oxidation of 2′, 7′-dichlorodihydrofluorescein (DCFH). Unfortunately, there may be alternative interpretations for these data, since the techniques used in both studies involved disruption of normal cellular structure. The hypothesis that an increased generation of oxidants in vivo plays a role in age-related tissue dysfunction is supported by studies in non-mammalian systems, where overexpression of CuZnSOD and catalase caused an extension of lifespan in Drosophila melanogaster (Orr & Sohal 1994) and by the description of extended lifespan in Caenorhabditis elegans treated with a MnSOD and catalase mimetic (Melov et al. 2000).
The apparent inconsistency between the large increase in ROS generation during exercise and the lack of a deleterious effect of muscle activity on muscle ageing appears to be due to the ability of skeletal muscle to adapt to the ROS generated during exercise by increasing the expression of protective proteins (McArdle & Jackson 2000). An increase in these proteins helps protect the tissue against subsequent exposure to exercise-induced increases in ROS generation (McArdle et al. 2004a). This ability to respond to oxidative stress does not appear to be maintained in ageing animals and humans (Rao et al. 1999; Vasilaki et al. 2002), but surprisingly is associated with an increase in the resting SOD, catalase, glutathione peroxidase (GPx) and glutathione reductase activities in the muscle of aged compared with adult mice (Leeuwenburgh et al. 1994). Muscle nNOS activities have been reported to be either elevated (Capanni et al. 1998) or decreased (Richmonds et al. 1999) in aged animals. Whether these changes are associated with any aberrant NOS activity in old muscle is also unclear although there is some evidence for a relocation of nNOS to the muscle cytosol in aged animals (Cappani et al. 1998).
The net effect of these changes in ROS activity in aged muscle is unclear as is the potential for any amelioration of age-related muscle dysfunction by reduction or modification of ROS levels. Despite this, our recent data indicate that transgenic approaches to express high levels of a protein that protects against oxidative damage (HSP70) can improve muscle function in old mice (McArdle et al. 2004b).
Acknowledgments
The author would like to thank his multiple co-workers and collaborators for their extensive contributions to the work described and to thank the Wellcome Trust, BBSRC, Research into Ageing and the United States National Institute on Aging for generous financial support.
Footnotes
One contribution of 18 to a Theme Issue ‘Reactive oxygen species in health and disease’.
References
- Balon T.W, Nadler J.L. Nitric oxide release is present from incubated skeletal muscle preparations. J. Appl. Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- Bejma J, Ji L.L. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J. Appl. Physiol. 1999;87:465–470. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- Bender A.T, Silverstein A.M, Demady D.R, Kanelakis K.C, Noguchi S, Pratt W.B, Osawa Y. Neuronal nitric-oxide synthase is regulated by the Hsp90-based chaperone system in vivo. J. Biol. Chem. 1999;274:1472–1478. doi: 10.1074/jbc.274.3.1472. 10.1074/jbc.274.3.1472 [DOI] [PubMed] [Google Scholar]
- Brenman J.E, Chao D.S, Xia H, Aldape K, Bredt D.S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. 10.1016/0092-8674(95)90471-9 [DOI] [PubMed] [Google Scholar]
- Capanni C, Squarzoni S, Petrini S, Villanova M, Muscari C, Maraldi N.M, Guarnieri C, Caldarera C.M. Increase of neuronal nitric oxide synthase in rat skeletal muscle during ageing. Biochem. Biophys. Res. Commun. 1998;245:216–219. doi: 10.1006/bbrc.1998.8404. 10.1006/bbrc.1998.8404 [DOI] [PubMed] [Google Scholar]
- Chang W.J, et al. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc. Natl Acad. Sci. USA. 1996;93:9142–9147. doi: 10.1073/pnas.93.17.9142. 10.1073/pnas.93.17.9142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino F, Luscher T.F. Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc. Res. 1999;43:274–278. doi: 10.1016/s0008-6363(99)00134-0. 10.1016/S0008-6363(99)00134-0 [DOI] [PubMed] [Google Scholar]
- Cosentino F, Patton S, d'Uscio L.V, Werner E.R, Werner-Felmayer G, Moreau P, Malinski T, Luscher T.F. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J. Clin. Invest. 1998;101:1530–1537. doi: 10.1172/JCI650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino F, et al. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- Crosbie R.H, Barresi R, Campbell K.P. Loss of sarcolemma nNOS in sarcoglycan-deficient muscle. FASEB J. 2002;16:1786–1791. doi: 10.1096/fj.02-0519com. 10.1096/fj.02-0519com [DOI] [PubMed] [Google Scholar]
- Davies K.J, Quintanilha A.T, Brooks G.A, Packer L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. 10.1016/S0006-291X(82)80124-1 [DOI] [PubMed] [Google Scholar]
- de Grey A.D. The reductive hotspot hypothesis: an update. Arch. Biochem. Biophys. 2000;373:295–301. doi: 10.1006/abbi.1999.1509. 10.1006/abbi.1999.1509 [DOI] [PubMed] [Google Scholar]
- Dillard C.J, Litov R.E, Savin W.M, Dumelin E.E, Tappel A.L. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J. Appl. Physiol. 1978;45:927–932. doi: 10.1152/jappl.1978.45.6.927. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2001;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Drummond G.R, Cai H, Davis M.E, Ramasamy S, Harrison D.G. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ. Res. 2000;86:347–354. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- Durbeej M, Campbell K.P. Muscular dystrophies involving the dystrophin–glycoprotein complex: an overview of current mouse models. Curr. Opin. Genet. Dev. 2002;12:349–361. doi: 10.1016/s0959-437x(02)00309-x. 10.1016/S0959-437X(02)00309-X [DOI] [PubMed] [Google Scholar]
- Fukai T, Siegfried M.R, Ushio-Fukai M, Cheng Y, Kojda G, Harrison D.G. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J. Clin. Invest. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G. Cellular and molecular aspects of adaptation in skeletal muscle. In: Komi P.V, editor. Strength and power in sport. Blackwell Science; Oxford, UK: 1994. pp. 211–229. [Google Scholar]
- Grozdanovic Z. NO message from muscle. Microsc. Res. Tech. 2001;55:148–153. doi: 10.1002/jemt.1165. 10.1002/jemt.1165 [DOI] [PubMed] [Google Scholar]
- Haddad J.J. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell. Signal. 2002;14:879–897. doi: 10.1016/s0898-6568(02)00053-0. 10.1016/S0898-6568(02)00053-0 [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J.M.C. Oxford University Press; Oxford, UK: 1989. Free radical biology and medicine. [Google Scholar]
- Hawley J.A. Adaptations of skeletal muscle to prolonged intense endurance training. Clin. Exp. Pharmacol. Physiol. 2002;29:218–222. doi: 10.1046/j.1440-1681.2002.03623.x. 10.1046/j.1440-1681.2002.03623.x [DOI] [PubMed] [Google Scholar]
- Hirschfield W, Moody M.R, O'Brien W.E, Gregg A.R, Bryan R.M, Jr, Reid M.B. Nitric oxide release and contractile properties of skeletal muscles from mice deficient in type III NOS. Am. J. Physiol. (Regul. Integr. Comp. Physiol.) 2000;278:R95–R100. doi: 10.1152/ajpregu.2000.278.1.R95. [DOI] [PubMed] [Google Scholar]
- Hwang J, Wang J, Morazzoni P, Hodis H.N, Sevanian A. The phytoestrogen equol increases nitric oxide availability by inhibiting superoxide production: an antioxidant mechanism for cell-mediated LDL modification. Free Radic. Biol. Med. 2003;34:1271–1282. doi: 10.1016/s0891-5849(03)00104-7. 10.1016/S0891-5849(03)00104-7 [DOI] [PubMed] [Google Scholar]
- Jackson M.J. Exercise and oxygen radical production by muscle. In: Sen C.K, Packer L, Hanninen O, editors. Exercise and oxygen toxicity. 2nd edn. Elsevier; Amsterdam: 2000. pp. 57–68. [Google Scholar]
- Jackson, M. J., Jones, D. A. & Edwards, R. H. T. 1983 Vitamin E and skeletal muscle. In Ciba Foundation Symp. no. 101 ‘Biology of Vitamin E’, pp. 224–239, London: Pitman. [DOI] [PubMed]
- Jackson M.J, Edwards R.H.T, Symons M.C.R. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim. Biophys. Acta. 1985;847:185–190. doi: 10.1016/0167-4889(85)90019-9. 10.1016/0167-4889(85)90019-9 [DOI] [PubMed] [Google Scholar]
- Jackson M.J, et al. Antioxidants reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol. Aspects Med. 2002;23:209–285. doi: 10.1016/s0098-2997(02)00018-3. 10.1016/S0098-2997(02)00018-3 [DOI] [PubMed] [Google Scholar]
- Javesghani D, Magder S.A, Barreiro E, Quinn M.T, Hussain S.N. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am. J. Respir. Crit. Care Med. 2002;165:412–418. doi: 10.1164/ajrccm.165.3.2103028. [DOI] [PubMed] [Google Scholar]
- Ji L.L. Antioxidant enzyme response to exercise and aging. Med. Sci. Sports Exerc. 1993;25:225–231. [PubMed] [Google Scholar]
- Ji L.L, Gomez-Cabrera M.C, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. 10.1096/fj.04-1846com [DOI] [PubMed] [Google Scholar]
- Khassaf M, Child R.B, McArdle A, Brodie D.A, Esanu C, Jackson M.J. Time course of responses of human skeletal muscle to oxidative stress induced by non-damaging exercise. J. Appl. Physiol. 2001;90:1031–1036. doi: 10.1152/jappl.2001.90.3.1031. [DOI] [PubMed] [Google Scholar]
- Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths R.D, Brodie D.A, Jackson M.J. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J. Physiol. 2003;549:645–652. doi: 10.1113/jphysiol.2003.040303. 10.1113/jphysiol.2003.040303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobzik L, Stringer B, Balligand J.L, Reid M.B, Stamler J.S. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem. Biophys. Res. Commun. 1995;211:375–381. doi: 10.1006/bbrc.1995.1824. 10.1006/bbrc.1995.1824 [DOI] [PubMed] [Google Scholar]
- Koh T.J, Tidball J.G. Nitric oxide synthase inhibitors reduce sarcomere addition in rat skeletal muscle. J. Physiol. 1999;519:189–196. doi: 10.1111/j.1469-7793.1999.0189o.x. 10.1111/j.1469-7793.1999.0189o.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Sohal B.H, Weindruch R, Forster M.J, Sohal R.S. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic. Biol. Med. 1998;25:1089–1097. doi: 10.1016/s0891-5849(98)00144-0. 10.1016/S0891-5849(98)00144-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuwenburgh C, Fiebig R, Chandwaney R, Ji L.L. Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am. J. Physiol. 1994;267:R439–R445. doi: 10.1152/ajpregu.1994.267.2.R439. [DOI] [PubMed] [Google Scholar]
- McArdle A, Jackson M.J. Exercise, oxidative stress and ageing. J. Anat. 2000;197:539–541. doi: 10.1046/j.1469-7580.2000.19740539.x. 10.1046/j.1469-7580.2000.19740539.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle A, Van der Meulen J.H, Catapano M, Symons M.C.R, Faulkner J.A, Jackson M.J. Free radical activity following contraction-induced injury to the extensor digitorum longus muscle of rats. Free Radic. Biol. Med. 1999;26:1085–1091. doi: 10.1016/s0891-5849(98)00317-7. 10.1016/S0891-5849(98)00317-7 [DOI] [PubMed] [Google Scholar]
- McArdle A, Pattwell D, Vasilaki A, Griffiths R.D, Jackson M.J. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am. J. Physiol. (Cell Physiol.) 2001;280:C621–C627. doi: 10.1152/ajpcell.2001.280.3.C621. [DOI] [PubMed] [Google Scholar]
- McArdle F, Spiers S, Aldemir H, Vasilaki A, Beaver A, Iwanejko L, McArdle A, Jackson M.J. Preconditioning of skeletal muscle against contraction-induced damage: the role of adaptations to oxidants in mice. J. Physiol. 2004a;561:233–244. doi: 10.1113/jphysiol.2004.069914. 10.1113/jphysiol.2004.069914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle A, Dillmann W.H, Mestril R, Faulkner J.A, Jackson M.J. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 2004b;18:355–357. doi: 10.1096/fj.03-0395fje. [DOI] [PubMed] [Google Scholar]
- McArdle A, et al. Role of mitochondrial superoxide dismutase in contraction-induced generation of reactive oxygen species in skeletal muscle extracellular space. Am. J. Physiol. Cell Physiol. 2004c;286:C1152–C1158. doi: 10.1152/ajpcell.00322.2003. 10.1152/ajpcell.00322.2003 [DOI] [PubMed] [Google Scholar]
- Melov S, Shoffner J.M, Kaufman A, Wallace D.C. Marked increase in the number and variety of mitochondrial DNA rearrangements in aging human skeletal muscle. Nucleic Acids Res. 1995;23:4122–4126. doi: 10.1093/nar/23.20.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. 10.1126/science.289.5484.1567 [DOI] [PubMed] [Google Scholar]
- Miura H, Bosnjak J.J, Ning G, Saito T, Miura M, Gutterman D.D. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ. Res. 2003;92:e31–e40. doi: 10.1161/01.res.0000054200.44505.ab. 10.1161/01.RES.0000054200.44505.AB [DOI] [PubMed] [Google Scholar]
- Morre D.M, Lenaz G, Morre D.J. Surface oxidase and oxidative stress propagation in aging. J. Exp. Biol. 2000;203:1513–1521. doi: 10.1242/jeb.203.10.1513. [DOI] [PubMed] [Google Scholar]
- Nader G.A, Esser K.A. Intracellular signalling specificity in skeletal muscle in response to different modes of exercise. J. Appl. Physiol. 2001;90:1936–1942. doi: 10.1152/jappl.2001.90.5.1936. [DOI] [PubMed] [Google Scholar]
- Nohl H. Involvement of free radicals in ageing: a consequence or cause of senescence. Br. Med. Bull. 1993;49:653–667. doi: 10.1093/oxfordjournals.bmb.a072638. [DOI] [PubMed] [Google Scholar]
- Orr W.C, Sohal R.S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- Ou Z, Ou J, Ackerman A.W, Oldham K.T, Pritchard K.A., Jr L-4F, an apolipoprotein A-1 mimetic, restores nitric oxide and superoxide anion balance in low-density lipoprotein-treated endothelial cells. Circulation. 2003;107:1520–1524. doi: 10.1161/01.cir.0000061949.17174.b6. 10.1161/01.CIR.0000061949.17174.B6 [DOI] [PubMed] [Google Scholar]
- Partridge T. Chapman & Hall; London: 1993. Molecular and cell biology of muscular dystrophy. [Google Scholar]
- Pattwell D.M, Jackson M.J. Contraction-induced oxidants as mediators of adaptation and damage in skeletal muscle. Exerc. Sport Sci. Rev. 2004;32:14–18. doi: 10.1097/00003677-200401000-00004. 10.1097/00003677-200401000-00004 [DOI] [PubMed] [Google Scholar]
- Pattwell D, McArdle A, Griffiths R.D, Jackson M.J. Measurement of free radical production by in vivo microdialysis during ischaemia-reperfusion injury to skeletal muscle. Free Radic. Biol. Med. 2001;30:979–985. doi: 10.1016/s0891-5849(01)00485-3. 10.1016/S0891-5849(01)00485-3 [DOI] [PubMed] [Google Scholar]
- Pattwell D.M, McArdle A, Morgan J.E, Patridge T.A, Jackson M.J. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic. Biol. Med. 2004;37:1064–1072. doi: 10.1016/j.freeradbiomed.2004.06.026. 10.1016/j.freeradbiomed.2004.06.026 [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Ordway G.A, Saltin B, Neufer P.D. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am. J. Physiol. (Endocrinol. Metab.) 2000;279:E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- Porter M.M, Vandervoort A.A, Lexell J. Aging of human muscle: structure, function and adaptability. Scand. J. Med. Sci. Sports. 1995;5:129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Pou S, Keaton L, Surichamorn W, Rosen G.M. Mechanism of superoxide generation by neuronal nitric-oxide synthase. J. Biol. Chem. 1999;274:9573–9580. doi: 10.1074/jbc.274.14.9573. 10.1074/jbc.274.14.9573 [DOI] [PubMed] [Google Scholar]
- Rao D.V, Watson K, Jones G.L. Age-related attenuation in the expression of the major heat shock proteins in human peripheral lymphocytes. Mech. Ageing Dev. 1999;107:105–118. doi: 10.1016/s0047-6374(98)00143-2. 10.1016/S0047-6374(98)00143-2 [DOI] [PubMed] [Google Scholar]
- Reid M.B. Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med. Sci. Sports Exerc. 2001;33:371–376. doi: 10.1097/00005768-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Reid M.B, Shoji T, Moody M.R, Entman M.L. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J. Appl. Physiol. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- Reznick A.Z, Packer L, Sen C.K, Holloszy J.O, Jackson M.J, editors. Oxidative stress in skeletal muscle. Birkhauser Verlag; Basel: 1998. [Google Scholar]
- Richmonds C.R, Boonyapisit K, Kusner L.L, Kaminski H.J. Nitric oxide synthase in aging rat skeletal muscle. Mech. Ageing Dev. 1999;109:177–189. doi: 10.1016/s0047-6374(99)00035-4. 10.1016/S0047-6374(99)00035-4 [DOI] [PubMed] [Google Scholar]
- Roberts C.K, Barnard R.J, Jasman A, Balon T.W. Acute exercise increases nitric oxide synthase activity in skeletal muscle. Am. J. Physiol. 1999;277:E390–E394. doi: 10.1152/ajpendo.1999.277.2.E390. [DOI] [PubMed] [Google Scholar]
- Schoneich C. Reactive oxygen species and biological aging: a mechanistic approach. Exp. Gerontol. 1999;34:19–34. doi: 10.1016/s0531-5565(98)00066-7. 10.1016/S0531-5565(98)00066-7 [DOI] [PubMed] [Google Scholar]
- Sohal R.S, Ku H.H, Agarwal S, Forster M.J, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. 10.1016/0047-6374(94)90104-X [DOI] [PubMed] [Google Scholar]
- Song Y, Cardounel A.J, Zweier J.L, Xia Y. Inhibition of superoxide generation from neuronal nitric oxide synthase by heat shock protein 90: implications in NOS regulation. Biochemistry. 2002;41:10 616–10 622. doi: 10.1021/bi026060u. 10.1021/bi026060u [DOI] [PubMed] [Google Scholar]
- Stamler J.S, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- Stralin P, Jacobsson H, Marklund S.L. Oxidative stress, NO* and smooth muscle cell extracellular superoxide dismutase expression. Biochim. Biophys. Acta. 2003;1619:1–8. doi: 10.1016/s0304-4165(02)00419-1. [DOI] [PubMed] [Google Scholar]
- Tidball J.G, Lavergne E, Lau K.S, Spencer M.J, Stull J.T, Wehling M. Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. Am. J. Physiol. 1998;275:C260–C266. doi: 10.1152/ajpcell.1998.275.1.C260. [DOI] [PubMed] [Google Scholar]
- Tidball J.G, Spencer M.J, Wehling M, Lavergne E. Nitric-oxide synthase is a mechanical signal transducer that modulates talin and vinculin expression. J. Biol. Chem. 1999;274:33 155–33 160. doi: 10.1074/jbc.274.46.33155. 10.1074/jbc.274.46.33155 [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Salvador C, Yang H, Huang T.T, Epstein C.J, Richardson A. Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch. Biochem. Biophys. 1999;363:91–97. doi: 10.1006/abbi.1998.1060. 10.1006/abbi.1998.1060 [DOI] [PubMed] [Google Scholar]
- Vasilaki A, Jackson M.J, McArdle A. Attenuated heat shock response of aged skeletal muscle to exercise. Muscle Nerve. 2002;25:902–905. doi: 10.1002/mus.10094. 10.1002/mus.10094 [DOI] [PubMed] [Google Scholar]
- Wehling M, Spencer M.J, Tidball J.G. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J. Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. 10.1083/jcb.200105110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widegren U, et al. Divergent effects of exercise on metabolic and mitogenic signalling pathways in human skeletal muscle. FASEB J. 1998;12:1379–1389. doi: 10.1096/fasebj.12.13.1379. [DOI] [PubMed] [Google Scholar]
- Wretman C, Lionikas A, Widegren U, Lannergren J, Westerblad H, Henriksson J. Effects of concentric and eccentric contractions on phosphorylation of MAPK(erk1/2) and MAPK(p38) in isolated rat skeletal muscle. J. Physiol. 2001;535:155–164. doi: 10.1111/j.1469-7793.2001.00155.x. 10.1111/j.1469-7793.2001.00155.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Dawson V.L, Dawson T.M, Snyder S.H, Zweier J.L. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc. Natl Acad. Sci. USA. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. 10.1073/pnas.93.13.6770 [DOI] [PMC free article] [PubMed] [Google Scholar]