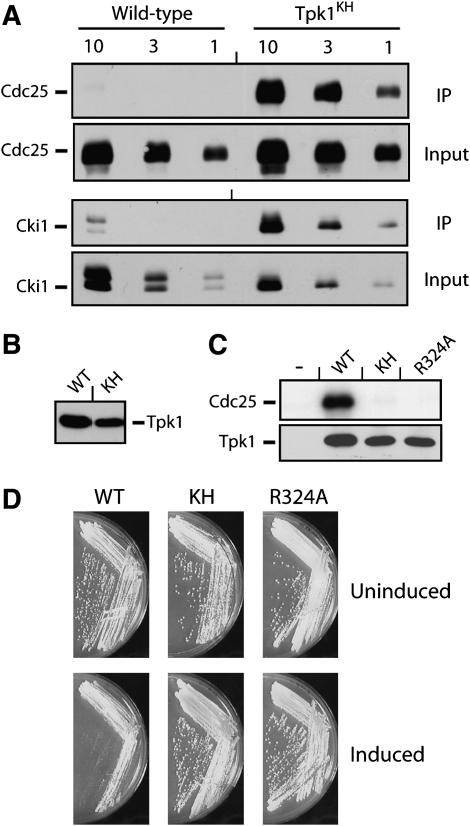
Figure 1.—
The Tpk1KH variant, but not the wild-type Tpk1, was physically associated with known PKA substrates. (A) The Tpk1KH variant was detected in immunoprecipitates with two PKA substrates, Cdc25 and Cki1. An HA epitope-tagged version of either the wild-type Tpk1 or the Tpk1KH variant was precipitated from cell extracts with an anti-HA affinity matrix. The bound substrate was eluted, separated on an SDS-polyacrylamide gel, and transferred to a nitrocellulose membrane. The relative amount of substrate present was then assessed by Western immunoblotting with antibodies specific for the myc epitope on the particular substrate present, either Cdc25 or Cki1. Three different amounts of the final immunoprecipitates and original inputs were loaded onto the gels, corresponding to 1, 3, and 10 μl of the 30 and 1000 μl present, respectively. (B) A Western immunoblot showing the relative levels of the wild-type Tpk1 and Tpk1KH present in the immunoprecipitates shown in A. (C) The Tpk1KH and Tpk1R324A variants were defective for PKA kinase activity in vitro. A myc epitope-tagged Cdc25 protein was incubated with [γ-32P]ATP and the wild-type Tpk1, Tpk1KH, or Tpk1R324A for 30 min at 25°. The reaction products were separated on an SDS-polyacrylamide gel and the extent of phosphorylation was assessed by autoradiography. (Bottom) A Western immunoblot showing the relative levels of the Tpk1 enzymes present in the kinase reactions. (D) The overexpression of the wild-type Tpk1 resulted in a growth defect in yeast cells. Wild-type cells were transformed with plasmids expressing the wild-type Tpk1, Tpk1KH, or Tpk1R324A from the CUP1 promoter. The cells were then plated to YM minimal media containing either 0 (Uninduced) or 100 μM (Induced) CuSO4 and incubated at 30° for 3 days.