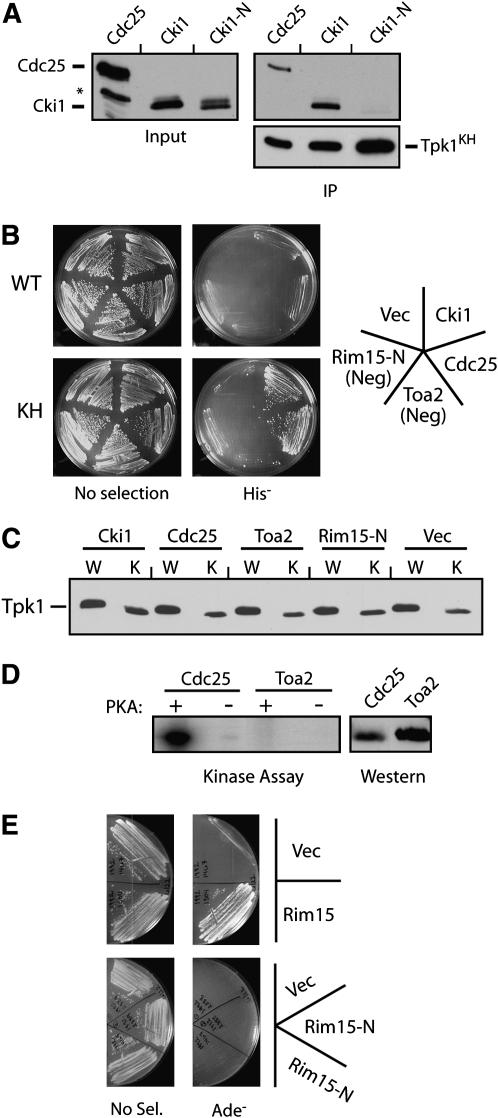
Figure 2.—
The interactions with the Tpk1KH variant were specific to PKA substrates. (A) The Tpk1KH variant did not associate with a fragment of Cki1 that lacks the sites of PKA phosphorylation. The relative amount of three different proteins, Cdc25 (codons 51–330), Cki1 (2–200), and Cki1-N (274–471), precipitating with the Tpk1KH variant was assessed as described in Figure 1A. The asterisk denotes a nonspecific protein band recognized by the α-myc epitope antibody. (B) A two-hybrid assay showing that the Tpk1KH variant interacted with the known substrates, Cdc25 and Cki1, but not with the nonsubstrates, Rim15-N and Toa2. The wild-type Tpk1 failed to interact with any of these test proteins. For these assays, the Tpk1 fusions to the Gal4 activation domain were under the control of the inducible promoter from the CUP1 gene (see materials and methods). Rim15-N (codons 361–657) was a negative control that was not phosphorylated by PKA. (C) A Western immunoblot showing the relative levels of the wild-type Tpk1 and Tpk1KH present in the two-hybrid strains shown in B. (D) Toa2 was not phosphorylated by PKA in vitro. Protein A fusions to Toa2 and a portion of Cdc25 (codons 51–330) were precipitated from yeast cell extracts and incubated with PKA and [γ-32P]ATP. The reaction products were separated on an SDS-polyacrylamide gel and the level of phosphorylation was assessed by autoradiography. (Right) A Western immunoblot showing the relative levels of the two substrates present in the kinase assays. (E) The Tpk1KH variant interacted with a Rim15 fragment containing the known sites of PKA phosphorylation (codons 1431–1671) but not with the Rim15-N fragment in a standard two-hybrid assay.