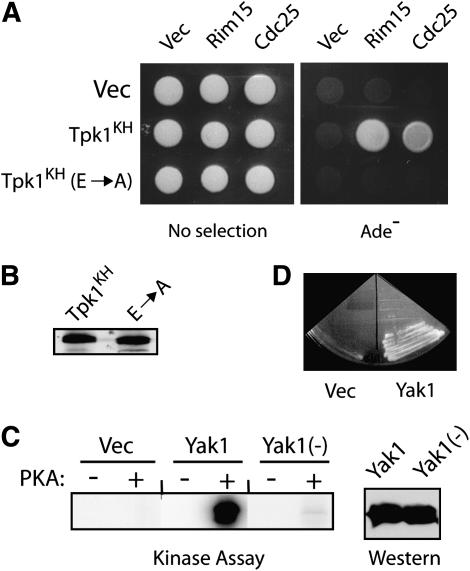
Figure 4.—
Tpk1KH binding to substrates required amino acid residues known to be important for the interaction between the wild-type PKA and its substrates. (A) A two-hybrid assay assessing the interactions between Tpk1KH variants and two substrates, Rim15 and Cdc25. The Tpk1KH (E → A) variant has replaced both E171 and E274 with an alanine residue. These glutamic acid residues have been shown previously to be important for Tpk1 interactions with peptide substrates (Gibbs and Zoller 1991a; Kemp et al. 1994). (B) A Western immunoblot showing the relative levels of the two Tpk1 enzymes present in the two-hybrid strains with Cdc25 as substrate. (C) A Yak1 fragment containing codons 243–361 is phosphorylated at a single site by PKA in vitro. Protein A fusions to Yak1 with either a wild-type (Yak1) or altered PKA site [Yak1(−)] were precipitated from yeast cell extracts and incubated with PKA and [γ-32P]ATP. The reaction products were separated on an SDS-polyacrylamide gel and the level of phosphorylation was assessed by autoradiography. (Right) A Western immunoblot showing the relative levels of the two Yak1 proteins present in the kinase assays. (D) A Yak1 fragment containing only a single site of PKA phosphorylation (codons 243–361) was recognized by the Tpk1KH variant in a two-hybrid assay.