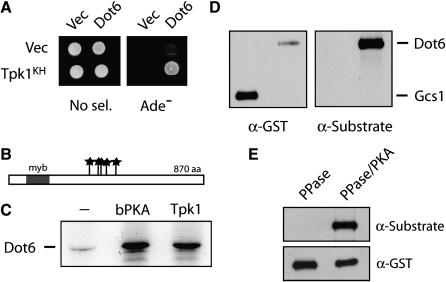
Figure 6.—
The Dot6 protein was identified as a candidate substrate for PKA. (A) Dot6 interacted with the Tpk1KH variant in a two-hybrid assay. (B) A schematic showing the positions of the five PKA consensus phosphorylation sites in the Dot6 protein; the sites are indicated by stars. A single myb domain, a DNA-binding motif originally identified in the c-myb oncoprotein, is present at the N-terminus of this protein (Singer et al. 1998). (C) The Dot6 protein was phosphorylated in vitro by the bovine PKA (bPKA) and the yeast Tpk1. (D) The Dot6 protein was recognized by an antibody specific for phosphorylated PKA sites. GST–Dot6 and GST–Gcs1 fusion proteins were precipitated with an anti-GST antibody, separated on an SDS-polyacrylamide gel, and transferred to a nitrocellulose membrane. The phosphorylation state of these proteins was then assessed by Western immunoblotting with an anti-PKA substrate antibody (Cell Signaling) that recognizes the phosphorylated forms of PKA target sites. The accompanying blot with an anti-GST antibody shows the relative levels of each fusion protein. (E) Dot6 recognition by the anti-PKA substrate antibody. The immunoprecipitated GST–Dot6 fusion was treated either with λ phosphatase only (PPase) or λ phosphatase and then bovine PKA and ATP (PPase/PKA). The reaction products were separated by SDS–PAGE electrophoresis, blotted to a nitrocellulose membrane, and analyzed by Western immunoblotting with either the anti-PKA substrate or anti-GST antibodies.