Figure 2. Pulse-chase analysis of transfected HEK 293 cells expressing normal or mutant αIIbβ3 receptors.
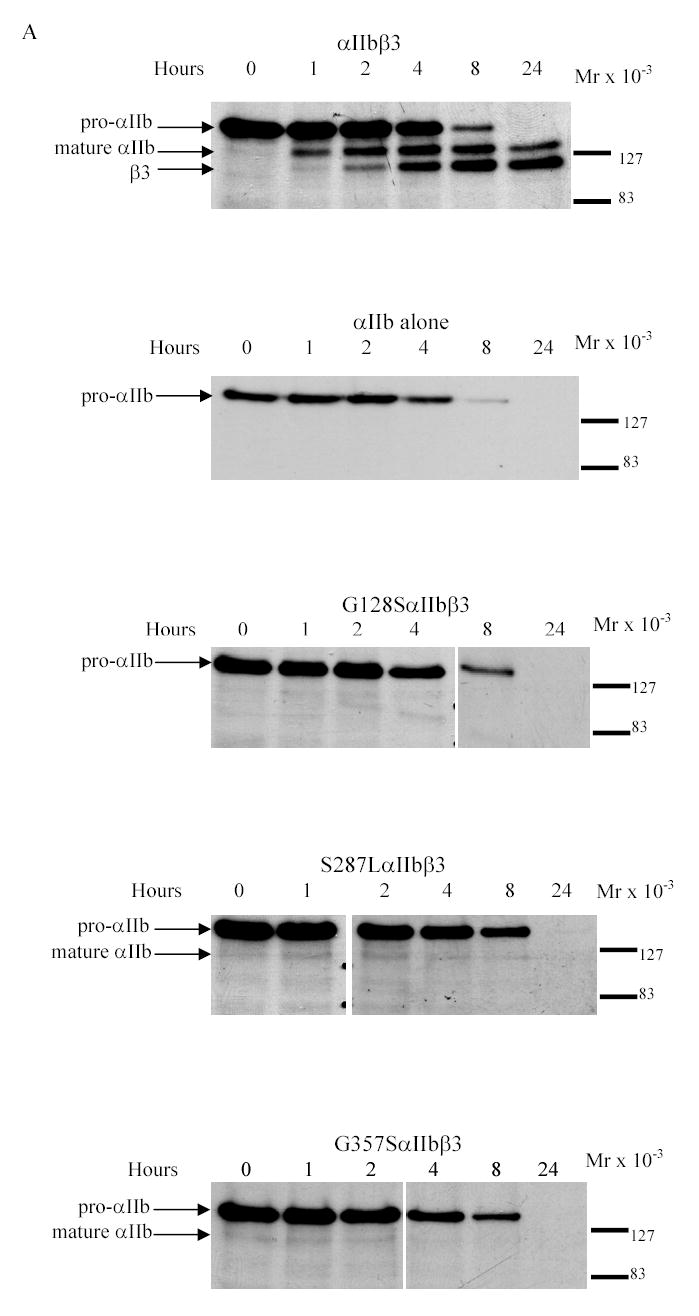
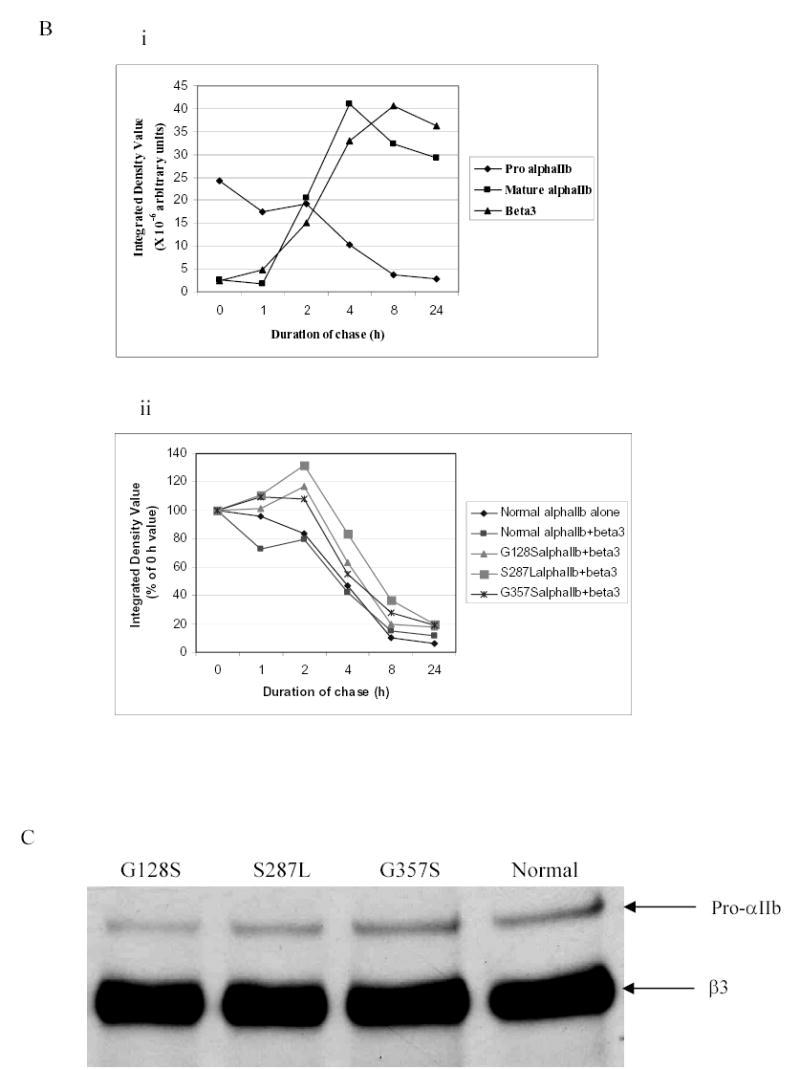
(A) Stable cell lines expressing normal or mutant αIIbβ3 were pulsed with 35S-methionine- and cysteine-containing medium for 15 min and chased in medium containing unlabeled methionine and cysteine for 0, 1, 2, 4, 8, and 24 h. The cell lysates were immunoprecipitated with an antibody to the V5-epitope tag on αIIb. Samples were electrophoresed under reduced conditions. Bands representing pro-αIIb, mature αIIb, and β3 are indicated by arrows. (B) (i) Kinetics of pro-αIIb, mature αIIb, and β3 in cell lines transfected with normal αIIb and β3 subunits as judged by band densities. There was a gradual decrease in pro-αIIb levels and this was mirrored by both an increase in mature αIIb over 24 h and an increase in β3 associated with αIIb. (ii) Kinetics of pro-αIIb in cell lines transfected with αIIb only or normal or mutant αIIbβ3 receptors. All three mutant αIIb subunits demonstrated similar rates of degradation, which were similar to the rates in the normal αIIbβ3 and αIIb only cell lines. (C) Immunoprecipitation with an antibody to β3 (7H2) 1 h after pulse-chase. Bands representing pro-αIIb and β3 are indicated by arrows.
