Figure 3. Co-localization of αIIb in the ER and Golgi apparatus in the transfected HEK 293 cells.
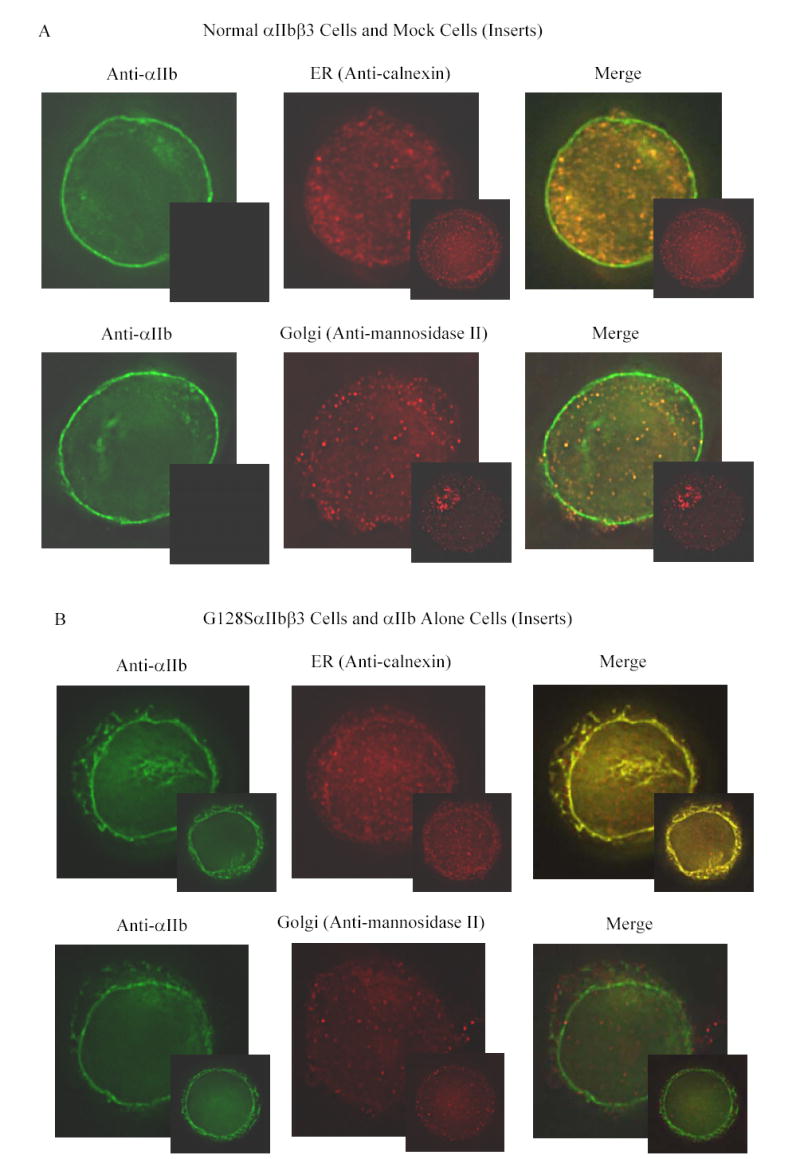
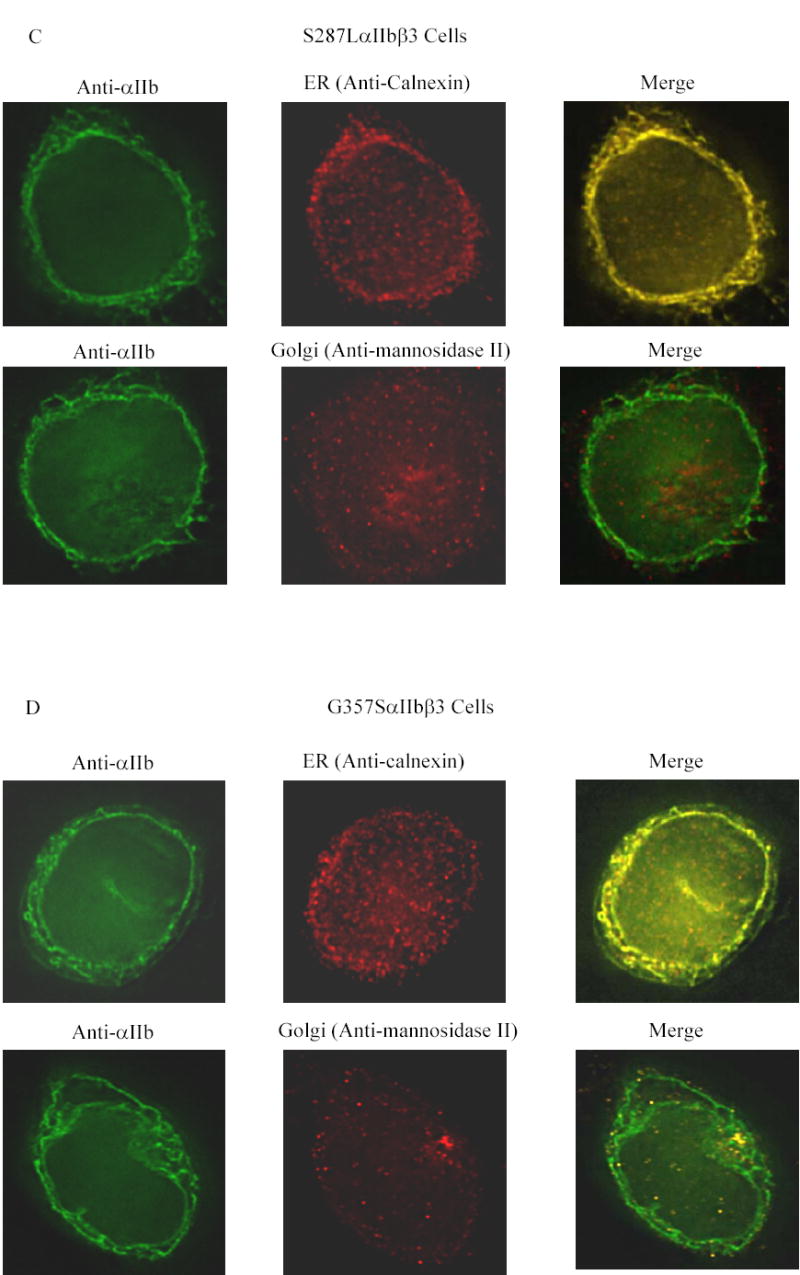
Transfected cells were labeled with an anti-αIIb antibody (green), and either an antibody to an ER component (calnexin) or a Golgi component (mannosidase II) (red). (A) Cells transfected with normal αIIbβ3 showed strong labeling of the cell surface, evident as a green outline, indicating normal αIIbβ3 expression. In addition, there is αIIb staining throughout the cell, some of which co-localizes with the ER marker and some with the Golgi marker, as indicated by the yellow color of the merged images. In contrast, mock transfected cells, shown here as inserts adjacent to the corresponding images of the normal cells, showed no αIIb staining either outside or inside the cell, but did show staining of the ER and Golgi. (B) Surface staining of αIIb was not observed in cells transfected with the G128SαIIbβ3 mutant. αIIb staining of the G128SαIIbβ3 mutant co-localized with the ER marker, indicated by the yellow color of the merged image, but not with the Golgi marker. The intensity of ER staining by anti-calnexin antibody in the G128SαIIbβ3 mutant cells was greater than in the normal αIIbβ3 cells. These results are comparable to cells transfected with αIIb alone (inserts). (C) S287LαIIbβ3 mutant cells demonstrated minimal amounts of αIIb on the surface. This mutant αIIb co-localized with the ER marker, but showed only faint co-localization with the Golgi marker, implying egress of only a small amount of S287LαIIbβ3 from the ER to Golgi. (D) In contrast, the G357SαIIbβ3 mutant cells demonstrated faint, but detectable surface labeling, and showed αIIb staining co-localizing with both the ER and the Golgi markers.
