Figure 5. Localization of the β-propeller mutations identified thus far in the αIIbβ3 crystal structure.

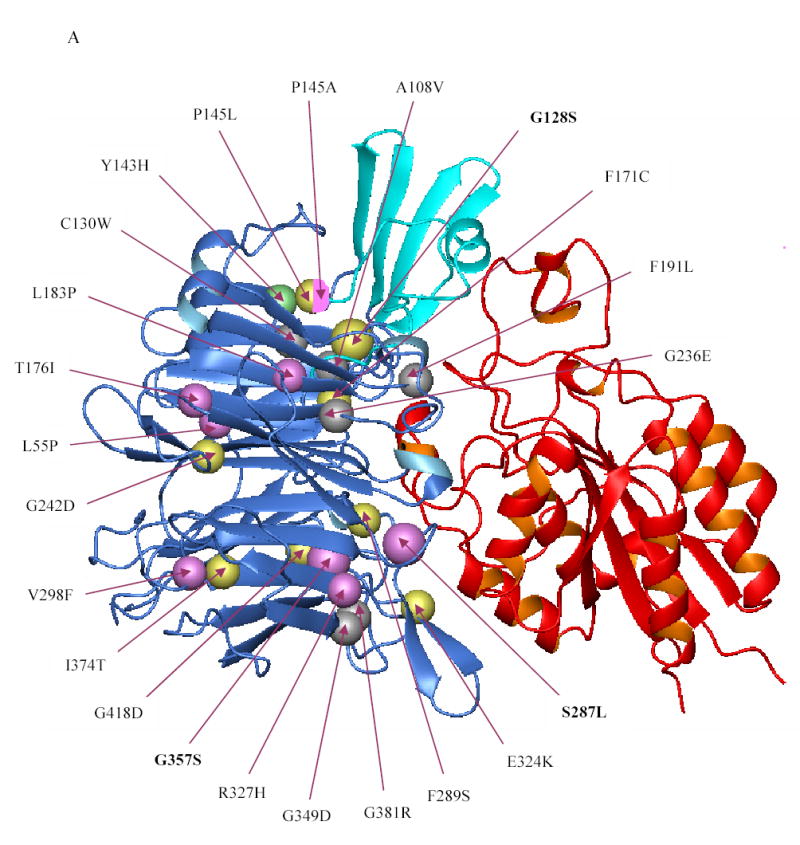
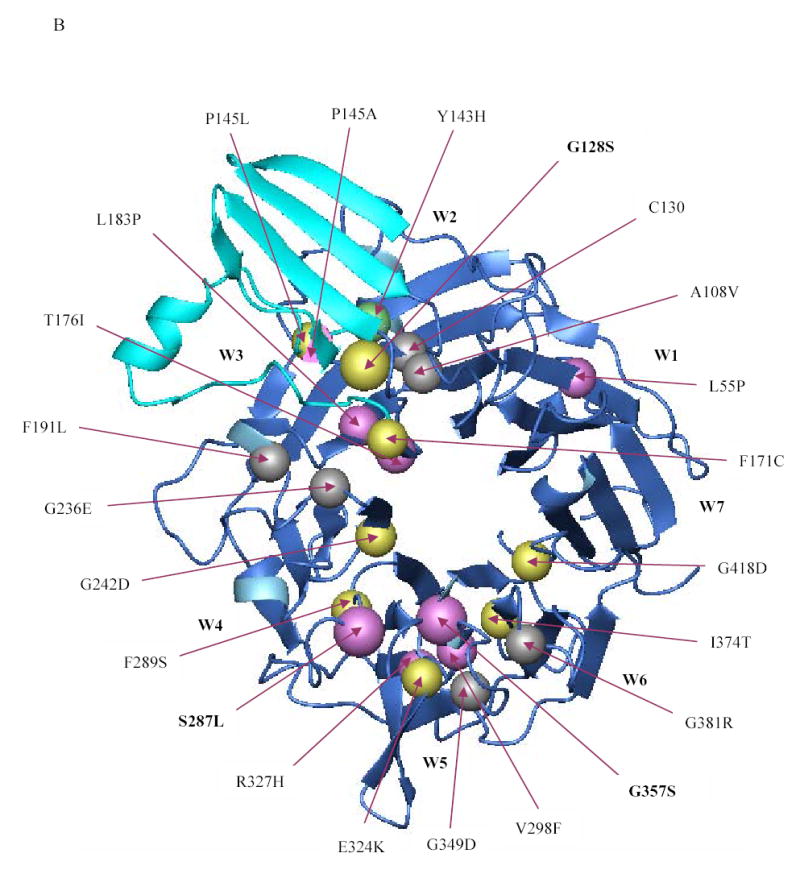
(A) Crystal structure of the interface between the β-propeller domain of αIIb (blue) and the βA domain of β3 (red) showing the location of the various β-propeller mutations reported thus far (including the ones reported in this paper). Mutations that result in no or low surface expression of αIIbβ3 are depicted in yellow and magenta, respectively. The Y143H mutation which affected ligand binding, but had little effect on surface expression, is shown in green, and lies adjacent to the cap region (cyan), implicated in ligand binding. Note that the mutations P145A, T176I, and L183P affected ligand binding as well as surface expression. The substitution of leucine or alanine for proline at position 145, respectively, resulted in no or low expression and this is depicted by a sphere that is half yellow and half magenta. Published mutations on which no data are available on either platelet surface expression or in vitro expression are depicted in gray. (B) Crystal structure of the β-propeller domain of αIIb as seen from the surface facing the β3 βA domain showing the various mutations published in the literature (including the ones in this paper). The central core formed by two concentric rings of aromatic residues is key to interacting with the βA domain of β3, which is not shown here.
