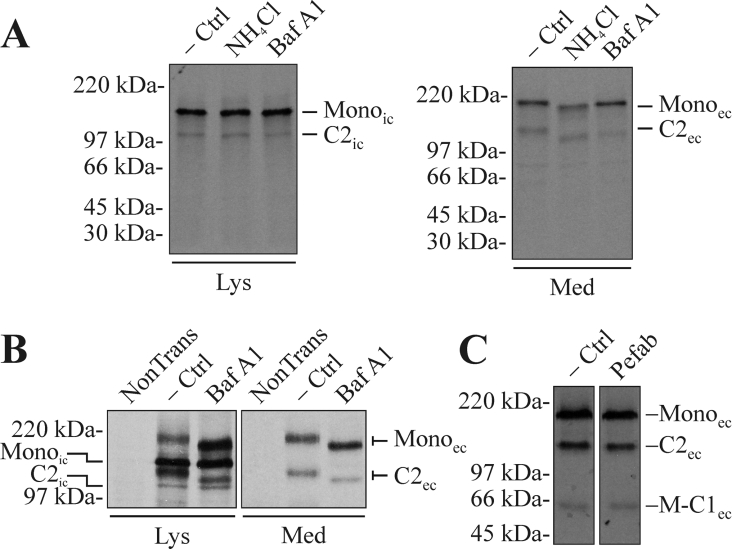
Figure 4. The cleavage in the MUC5AC C-terminus starts early in the secretory pathway, continues in the acidic parts of the late secretory pathway and does not involve serine protease activity.
(A) CHO-K1-pSM-MUC5AC-CH cells were pre-incubated with media with or without 25 mM ammonium chloride for 12 h. After starving the cells for 1 h, they were metabolically labelled with Redivue Pro-mix L-[35S] in vitro labelling mixture for 6 h. Both the starving and labelling steps were performed in the presence of the same concentration of ammonium chloride as during the pre-incubation. In the case where bafilomycin A1 was used as the neutralizing agent, 300 nM bafilomycin A1 was present during the starvation and labelling steps. Cell lysates and media were prepared, immunoprecipitated with α-myc mAb-coated magnetic beads and analysed by SDS/PAGE (3–10%, reducing conditions) and autoradiography. −Ctrl, non-treated cells; NH4Cl, ammonium chloride-treated cells; Baf A1, bafilomycin A1-treated cells. (B) LS 174T cells were transfected with the plasmid encoding the recombinant MUC5AC C-terminus. After 40 h, the cells were treated with bafilomycin A1 and cell lysates and media were immunoprecipitated and analysed as above. NonTrans, non-transfected cells. (C) CHO-K1-pSM-MUC5AC-CH cells were starved (1 h) and labelled (4 h) as above, in the presence of the serine protease inhibitor Pefabloc SC (200 μM). The spent cell-culture media was immunoprecipitated and analysed as above. Pefab, Pefabloc SC-treated cells. Lys, material from cell lysates; Med, material from spent cell-culture media. Mono represents the intact monomer, while M-C1 represents the N-terminal cleavage fragment and C2 the C-terminal one. The subscripts ‘ic’ and ‘ec’ indicate the intra- and extra-cellular forms of the protein respectively. Positions of molecular-mass standards are indicated to the left.