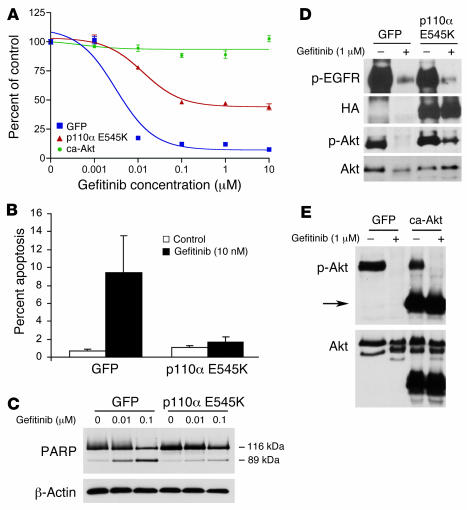
Figure 4. Downregulation of PI3K/Akt signaling is necessary for gefitinib to effectively induce apoptosis.
(A) Retroviral expression of HA-tagged oncogenic PI3K (p110α E545K) or ca-Akt into HCC827 cells conferred resistance to gefitinib. Expression constructs were introduced into HCC827 cells by retroviral infection, and the resulting cell lines were subjected to an MTS survival assay in increasing concentrations of gefitinib. (B) Expression of p110α E545K abrogated apoptosis induced by gefitinib. HCC827 cells expressing GFP or p110α E545K were treated with 10 nM gefitinib for 72 hours and assessed for apoptosis by analyzing the percent of cells in SubG1 by FACS analysis as previously described (30). (C) Cells were treated as in B, lysed, and assessed for PARP cleavage. Increasing gefitinib concentrations led to increased appearance of the cleaved 89-kDa fragment in HCC827 GFP cells compared with HCC827 p110α E545K cells. (D) p110α E545K led to persistent Akt activation. HCC827 cells expressing GFP or p110α E545K were exposed to 1 μM gefitinib for 12 hours prior to lysis. Note that expression of HA-tagged P110α E545K led to diminished but persistent p-Akt expression in the presence of gefitinib. (E) ca-Akt was not inhibited by gefitinib. HCC827 ca-Akt and HCC827 GFP cells were grown with or without 1 μM gefitinib for 12 hours and lysed for Western blot analysis. Arrow indicates ca-Akt that migrates at a lower molecular weight because it lacks the pleckstrin homology domain. Gefitinib inhibited the phosphorylation of the endogenous but not the myristoylated Akt.