Figure 1.
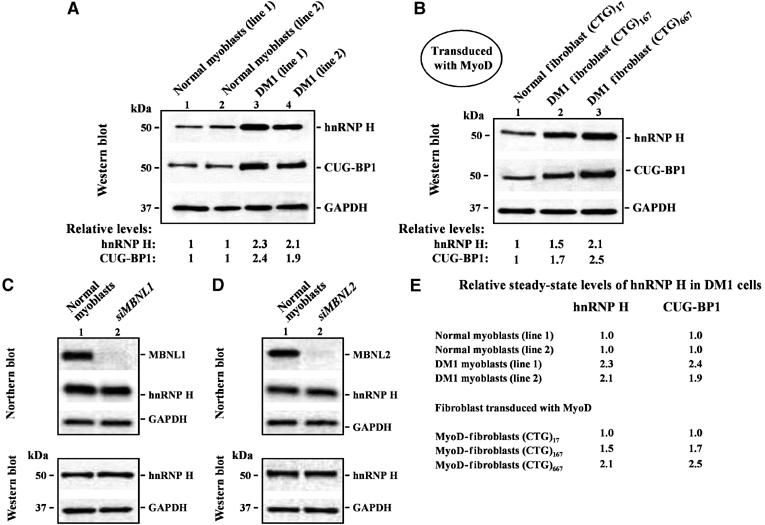
Steady-state hnRNP H levels are elevated in DM1 myoblasts by mechanisms unlinked to MBNL1 and MBNL2 loss. (A) Endogenous hnRNP H levels were measured in 10 μg of total protein from two normal and two DM1 myoblasts lines by Western blot analyses. The levels of hnRNP H in the DM1 myoblasts lines tested are ∼2.3- and ∼2.1-fold higher than that observed in normal myoblasts. CUG-BP1 levels in the DM1 myoblasts lines are ∼2.4- and ∼1.9-fold higher than that observed in the normal myoblast lines (n=3). (B) Fibroblasts containing 17, 167 and 667 CTG repeats were transduced with adenoviral vectors expressing both MyoD and GFP. Fluorescence microscopy study of fibroblasts 24 h after transduction demonstrated that ∼95% (±5%) of the transduced cells expressed GFP (data not shown). The levels of hnRNP H in the transduced DM1 fibroblasts were ∼1.5 fold (CTG)167 and ∼2.1 fold (CTG)667 elevated when compared with normal transduced fibroblasts (CTG)17 repeats (n=3). CUG-BP1 levels in the transduced DM1 fibroblasts were ∼1.7-fold (CTG)167 and ∼2.5-fold (CTG)667 higher than that observed in the normal transduced fibroblasts (CTG)17 repeats (n=3). (C, D) siRNA-mediated depletion of MBNL1 and MBNL2 in normal myoblasts does not result in increased steady state hnRNP H RNA or protein levels. Northern blot analyses demonstrate that MBNL1 or MBNL2 were ∼95 and ∼93% silenced, respectively. hnRNP H RNA and protein levels in normal myoblasts 5 days after transfection with siRNA directed against MBNL1 and MBNL2 were measured by Northern blot and Western blot analyses respectively. In both cases, the membranes were stripped and re-probed for GAPDH RNA and protein in parallel as an internal control. (E) Relative steady-state levels of hnRNP H and CUG-BP1 in DM1 cells when compared to normal controls is shown.
