Figure 5.
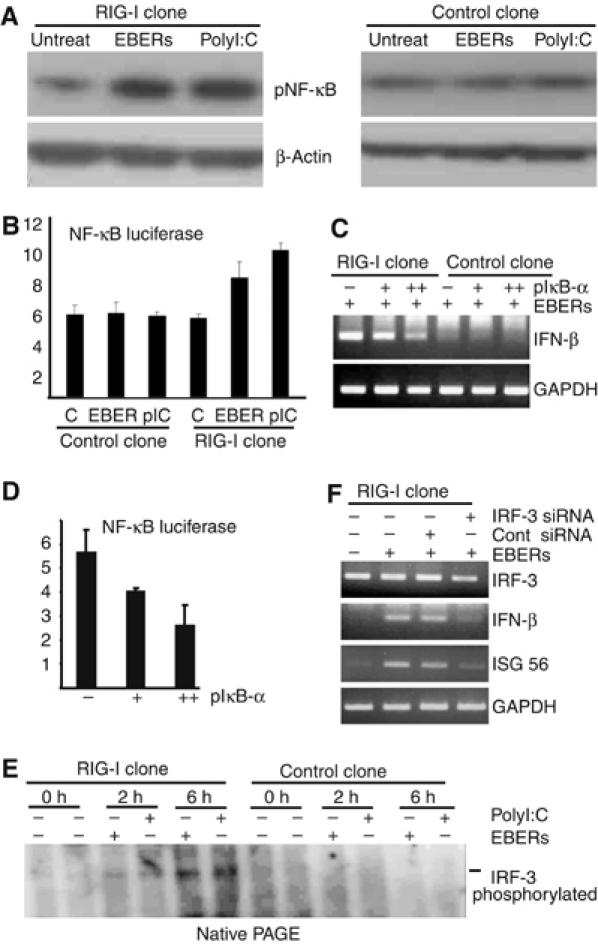
EBERs activate NF-κB and IRF-3. (A) Phosphorylation of NF-κB in RIG-I- or GFP-expressing stable clone was analyzed after they (5 × 106 cells each) were transfected with 30 μg of in vitro-synthesized EBERs (1:1) or polyI:C (positive control). After 6 h of transfection, whole-cell lysates (20 μg of protein for each sample) were subjected to immunoblot analysis for detection of phosphorylated NF-κB. (B) NF-κB reporter assay. NF-κB plasmid was transfected into both RIG-I-expressing Daudi cell clone and control clone and after 36 h of transfection, EBERs or polyI:C were transfected. After 12 h of EBER or polyI:C transfection, luciferase activity was measured in cell lysates (Figure 5B). Results are shown as the means±standard errors from three independent experiments. (C) Effect of IκB-α, a specific inhibitor of NF-κB, on EBER-induced phosphorylation of NF-κB. RIG-I-expressing stable clones (5 × 106 cells) were first transfected with 10 μg (+) or 20 μg (++) of IκB-α plasmid. After 36 h, they were further transfected with 30 μg of in vitro-synthesized EBERs (1:1), and expression of IFN-β was examined by RT–PCR after 8 h of transfection. (D) Inhibition of NF-κB activation by IκB-α plasmid. RIG-I-expressing stable clones (5 × 106 cells) were transfected with 10 μg of pNF-κB/luc (firefly luciferase) and 100 ng of pCMV/luc (Renilla luciferase as an internal control), along with 10 μg (+) or 20 μg (++) of IκB-α plasmid. After 36 h, luciferase activity was quantified in cell lysates using a Dual-Luciferase reporter assay system (Promega). Results are shown as the means±standard errors from three independent experiments. (E) Phosphorylation of IRF-3 in EBV-negative Daudi cells stably expressing RIG-I. RIG-I- or GFP- (5 × 106 cells each) expressing stable clones were transfected with 30 μg of in vitro-synthesized EBERs (15 μg each) or polyI:C (positive control). After 2 and 6 h of transfection, whole-cell lysates (30 μg of protein for each sample) were separated with native PAGE gel (8%) to analyze the phosphorylation of IRF-3 by EBERs or polyI:C. The blot was probed with anti-phosho-IRF-3 antibody. This antibody reacted with phosphorylated IRF-3, but not with unphosphorylated IRF-3. (F) RIG-I-expressing stable clone was treated with 100 nM of IRF-3 siRNA or control siRNA. After 24 h, cells were transfected with 30 μg of in vitro-synthesized EBERs, and 6 h later, expression of IRF-3, IFN-β, and ISG56 was examined by RT–PCR.
