Fig. 3.
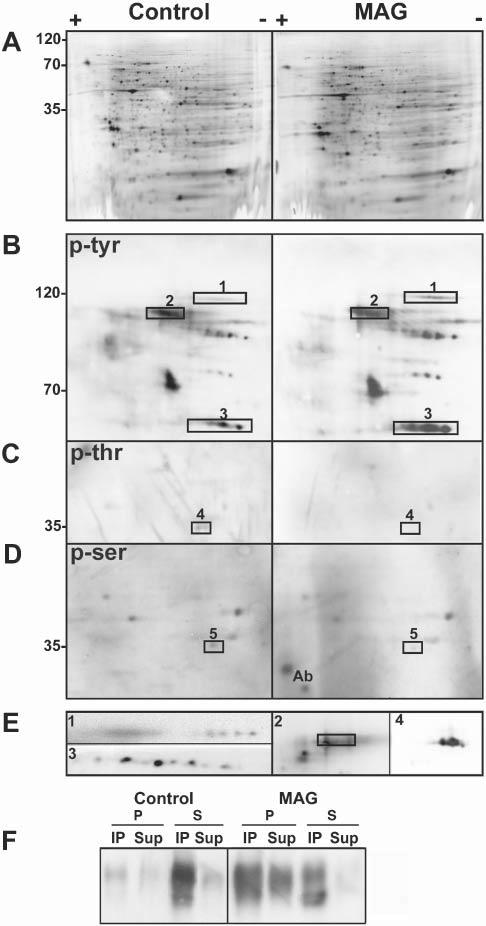
Specific proteins change their phosphorylation status upon antibody cross-linking of MAG. (A–E) Two-dimensional electrophoresis of cell lysates (300 μg total cell protein) from untreated, control OLs in culture and OLs treated with anti-MAG mAb (anti-MAG mAb, 1:100, 15 minutes; anti-mouse IgG, 1:500, 15 minutes) (MAG). Western blots are silver stained (A), and stained for anti-phosphotyrosine (p-tyr) (B), anti-phosphothreonine (p-thr) (C) and anti-phosphoserine (p-ser) (D). (E) Proteins that change their phosphorylation status upon MAG cross-linking identified by immunoblotting with specific antibodies (rectangles indicate position of these proteins in the respective phospho-immunoblot). 1, α-fodrin; 2, MAG, the rectangle encloses the pan-phosphotyrosine signal (2 in B). MAG overlaps with only the lower part of the phosphotyrosine signal because of the presence of other phosphoproteins in that region; 3, fyn; 4, Gβ; and 5, LDH. Ab: reactivity of some remaining antibody light chain. Images are enlarged in terms of pI and molecular weight in B–E compared to A. (F) Control and antibody-treated cells (MAG) were subjected to immunoprecipitation with anti-phosphotyrosine antibody followed by Western blot analysis of MAG. Cell lysates (20 μg) were extracted with TX-100 at 4°C and separated into pellets (P) and supernatants (S) by centrifugation. The entire yield in each fraction was used for immunoprecipitation (see Methods). The immunoprecipitated (IP) and non-immunoprecipitated (Sup) proteins were analyzed by Western blot using anti-MAG. Typical results of three independent experiments are shown.
