Abstract
The urethra is the main place of entry for sexually transmitted pathogens. However, there is little literature on the morphology of the urogenital system, principally the urethra and ducts of the sex accessory glands. The Mongolian gerbil is an insectivorous, herbivorous and monogamous rodent with nocturnal habits; it has been used successfully as a laboratory animal since the 1960s. Therefore, the objective of the present paper was to describe the structure and ultrastructure of the urethra and its relations to the ducts of the accessory sex glands of the Mongolian gerbil (Meriones unguiculatus), contributing to the understanding of the reproductive biology of the rodent and aiming to provide data for future experimental studies. Conventional techniques of light and scanning electron microscopy were utilized. The urethra and ducts of the accessory sex glands are similar to those of the albino rat and the mouse. However, there is variation in drainage type among accessory sex glands for the inner urethra. The ducts of the seminal vesicle, the ductus deferens, drain their contents independently into the ampullary duct that opens in the urethra. The ducts of the prostate, coagulating and bulbourethral glands drain their contents independently into the urethra.
Keywords: male urogenital system, morphology, rodents
Introduction
The male urethra constitutes the place of entry for sexually transmitted pathogens (Quayle et al. 1994). In the rat and the mouse, the urethra is divided into pelvic and penile portions (Hayes, 1965; Hummel et al. 1966; Hebel & Stromberg, 1976).
The origin of the distal part of the male urethra has been disputed by researchers, although there is general agreement on the development of the proximal part (Kanagasuntheram & Anandarajara, 1960). Embryologists have stated that the urethra within the navicular fossa was formed by an ectodermal surface, while the remainder of the urethra was formed by an endodermal surface. Despite the embryological and histological interest described above, the urethra has been given little attention (Nakano & Muto, 1989), in contrast to the abundant studies on the urinary bladder, ureter and kidney (Fulker et al. 1971; Kumagai, 1975; Kjaer et al. 1976; Newman & Hicks, 1977; Tannenbaum et al. 1978; Hayakawa, 1979; Nelson et al. 1979).
To date, the study of the structure of the sex ducts in mammals (including man and laboratory rodents), the accessory glands, has been reviewed only partially. The Mongolian gerbil (Meriones unguiculatus) is a rodent known for its suitability for laboratory use. By nature, it is a curious and friendly rodent, almost without odour, with monogamous behaviour and physiological mechanisms of corporal-water conservation, presenting spontaneous attacks of epilepsy and relative absence of natural illnesses. The purpose of the present paper was to describe the structure of the pelvic and penile urethra and their relationship to the ducts of the accessory sex glands, and to provide data for future experimental studies.
Materials and methods
Animals
Thirteen adult male gerbils (Meriones unguiculatus), aged 3 months and with an average weight of 90 g, were used. Gerbils were maintained under controlled temperature (23 °C) and lighting conditions (12: 12 h light–dark photoperiod, lights-out at 07:00 h). The experimental protocol followed the ethical principles in animal research adopted by the Brazilian College of Animal Experimentation.
Light microscopy (LM)
Nine adult male gerbils (Meriones unguiculatus), aged 3 months and with an average weight of 90 g, were used. The animals were anaesthetized with ether and perfused with Bouin‘s solution through the left ventricle according to the method of Sprando (1990). After the organs of the urogenital system, including the prostate, seminal vesicles, coagulating glands, urethra, urinary bladder, part of the ureters, ductus deferens and crus penis, had been isolated, collected and fixed in Bouin‘s solution for 24 h, they were washed in a solution of 70% alcohol, dehydrated in a graded ethanol series, embedded in paraplast and cut into 5–7-µm-thick sections. The paraplast blocks were cut so that the sections were either parallel or perpendicular to the urethra. The sections were mounted on glass slides, stained with Hematoxylin-Eosin (HE), Mallory‘s thricrome (MlT), Masson‘s thricrome (MaT), Calleja (C) and Azan-Heidenhain-Mallory (A). The sections were observed and photographed in a Zeiss Axiophot 2 light microscope.
Scanning electron microscopy (SEM)
Four adult male gerbils (Meriones unguiculatus), aged 3 months and with an average weight of 90 g, were used. The animals were anaesthetized with ether and perfused with glutaraldehyde solution through the left ventricle according to the method of Sprando (1990). Fragments of the pelvic and penile urethra were fixed in 2.5% glutaraldehyde in 0.1 m phosphate buffer (pH 7.2) for 3 h. Next, the fragments were post-fixed in 1% osmium tetroxide in 0.1 m phosphate buffer (pH 7.2) for 2 h in darkness. Subsequently, the fragments were dehydrated in graded ethanol. They were then submitted to critical point drying in liquid CO2 (Balzers CPD-010), coated with gold ions in SEM coating (Balzers MED 010) and examined under a Phillips FEM 515 SEM at 15 kV.
Results
Urethra
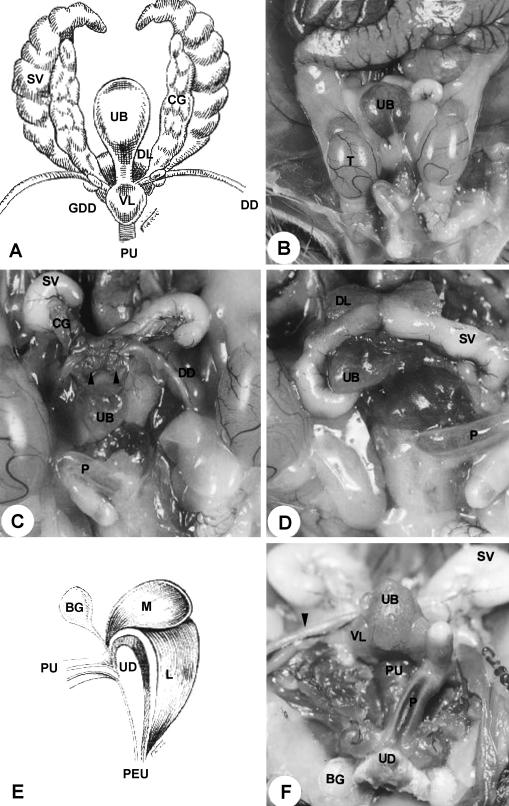
The urethra of the gerbil presented two portions: pelvic and penile. The pelvic urethra extended from an internal ostium in the urinary bladder to the level of the penile bulb. The penile urethra extended from the penile bulb to the external ostium of the urethra (Figs 1A,E,F and 4A). The pelvic and penile urethra were shown to be connected through the isthmus (Fig. 5B). The structure of the urethra varied in its different segments.
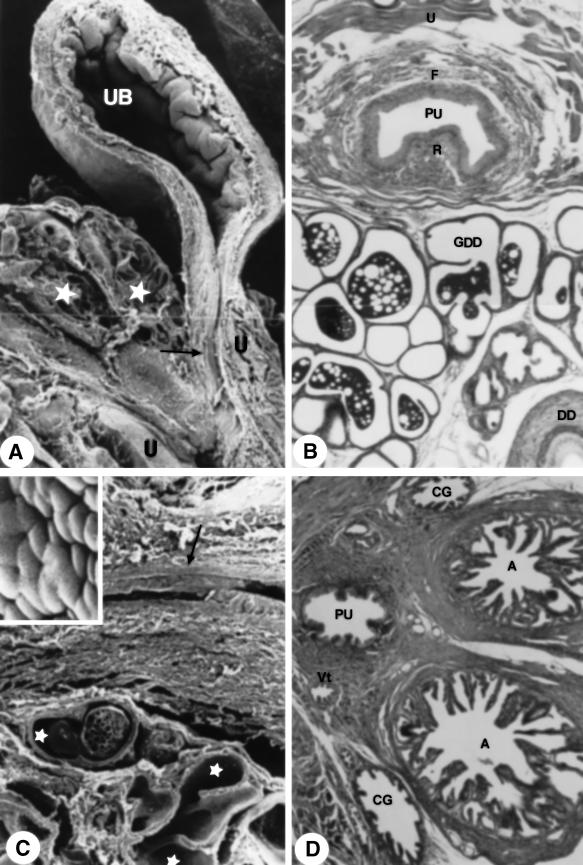
Fig. 1.
(A) Diagram of the ventral view of the sex accessory glands. (B) Male reproductive organs in situ. ×1.5. (C) Ventral view of the male urogenital system. ×2.6. (D) Male urogenital organs in situ. ×36. (E) Diagram of the penile bulb in the median section. (F) Ventral view of the male urogenital system. Arrowhead, ductus deferens. ×2.0. For abbreviations, see text.
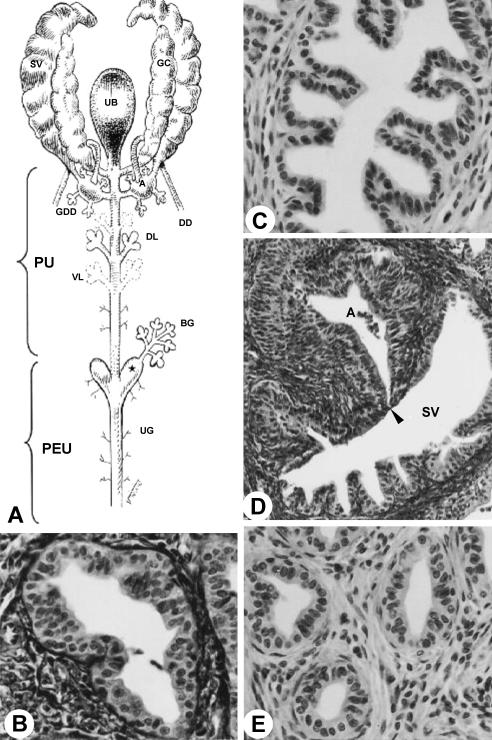
Fig. 4.
(A) Diagram of the ventral view of the sex accessory gland, ducts and urethra. Star, urethral diverticle. (B) Dorsal prostatic duct. MlT stain. ×1750. (C) Duct of the coagulating gland. HE stain. ×2285. (D) Opening (arrowhead) of the duct of the seminal vesicle (SV) into ampullary duct (A). MlT stain. ×895. (E) Ventral prostatic duct. HE stain. ×1644. For abbreviations, see text.
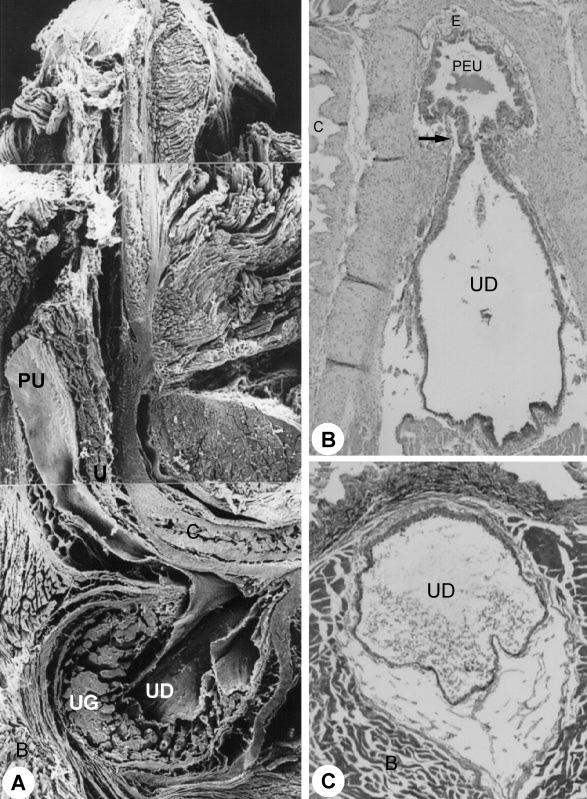
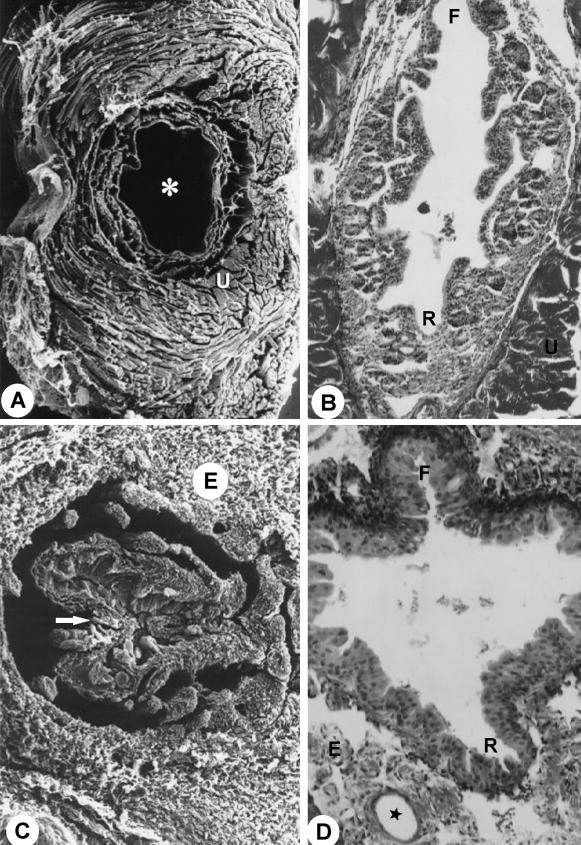
Fig. 5.
Sagittal section through the penile bulb. ×25. (B) Transition between pelvic and penile urethra (region of the penile bulb). Arrow, isthmus of the urethra. HE stain. ×280. (C) Urethral diverticle (UD). MaT stain. ×266. For abbreviations, see text.
Next to the neck of the urinary bladder, the pelvic urethra presented transitional epithelium with basal, intermediate and superficial cells. The basal presented cubic or prismatic forms, whereas the intermediate were polyhedral forms of various sizes and the superficial irregular polygonal forms (Fig. 6A). The pelvic urethra presented a thick layer of urethral muscle, surrounding it like a belt and finishing between the ischiocavernosum muscle and penile bulb (Figs 2A,B and 5A). In the stromal tissue thin blood vessels and muscle fibres were observed.
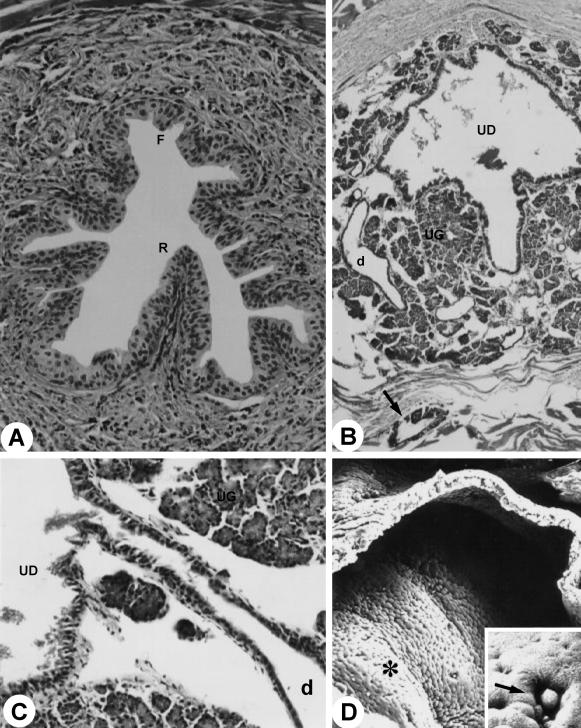
Fig. 6.
Pelvic urethra. F, floor; R, roof. MlT stain. ×1000. (B) Duct (d) of the urethral gland (UG); arrow, duct of the bulbourethral gland. HE stain. ×228. (C) Opening of the duct of the urethral gland (UG) into the urethral diverticle (UD). HE stain. ×900. (D) Section through urethral diverticle (asterisk). ×185. Inset: Arrow, detail of the opening duct of the urethral gland into the urethral diverticle. ×970. For abbreviations, see text.
Fig. 2.
(A) Sagittal section through urinary bladder. Stars, glandular tissue; arrow, pelvic urethra. ×20. (B) Cross-section through pelvic urethra. MlT stain. ×455. (C) Glandular tissue detail (stars) and pelvic urethra (arrow). ×465. Inset: epithelium surface of the pelvic urethra. The superficial cells are irregularly polygonal. ×1110. (D) Cross-section through the pelvic urethra. MlT stain. ×202. For abbreviations, see text.
The portion of the pelvic urethra next to the penile bulb presented stratified cylindrical epithelium, with occasional areas of pseudostratified cylindrical epithelium. The proper lamina and the muscle layer (urethral muscle) were shown to be similar to the origin (Fig. 7A,B).
Fig. 7.
(A) Cross-section through pelvic urethra (asterisk). U, urethral muscle. ×40. (B) Cross-section through roof (R) and floor (F) of the pelvic urethra. MaT stain. ×450. (C) Cross-section through penis. Arrow, penile urethra. ×200. (D) Cross-section through roof and floor of the penile urethra. Star, deep penile artery. MaT stain. ×386. For abbreviations, see text.
At the ischiadic arch the pelvic urethra enters in the urethral diverticle, an expansion of the penile urethra with a heart-shaped form and bilateral symmetry. The diverticle mucosa, with longitudinal folds allowing its dilation, presented stratified columnar epithelium and rich proper lamina in elastic, collagenous and smooth muscle fibres and blood vessels. Adenomeres of the urethral gland were connected to the diverticle, unloading its secretions in the lumen (Fig. 6B–D).
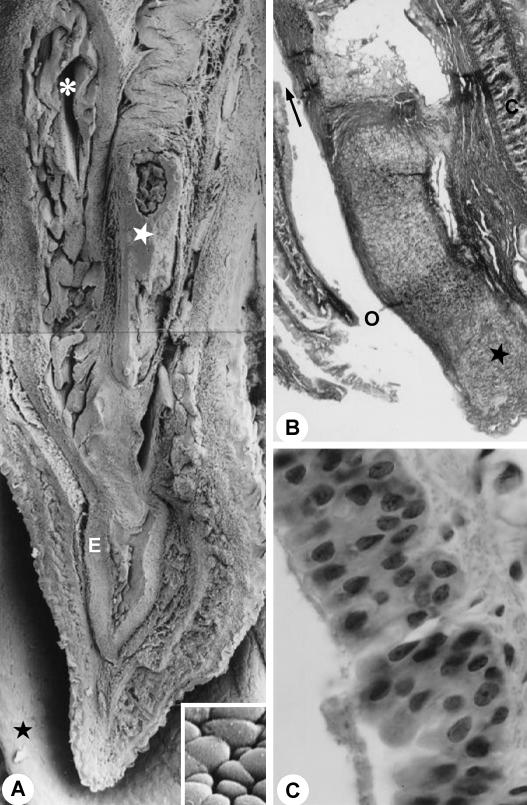
Further on, the urethra returns at an acute angle and is narrowed significantly, forming the isthmus; it continues in the spongiosum corpus as the penile urethra (Figs 1E and 5A). The penile urethra crosses the spongiosum corpus and the gland penis. It runs ventrally and finishes in the gland, under the penile bone (Fig. 8A,B), opening up in the distal extremity of the penis in the external ostium of the urethra. The penile urethra has a smaller diameter (Fig. 7C,D) than the pelvic urethra. The epithelium presented stratified columns (Fig. 8C) with a varying number of cellular layers.
Fig. 8.
(A) Sagittal section through body of the penis. Asterisk, penile urethra; white star, penile bone; E, corpus spongiosum; black star, prepuce. ×60. Inset: detail of the surface of the epithelium of the penile urethra. The cells show a paving-stone appearance. ×785. (B) Longitudinal section of the glans penis. Arrow, opening of the penile urethra; star, penile bone. C stain. ×428. (C) Epithelium stratified columnar. HE stain. ×4400. For abbreviations, see text.
Ducts of the sex accessory glands
The glandular portion of the urogenital system is formed by the seminal vesicles, coagulating glands, ductus deferens, bulbourethral and urethral glands and also prostate involving the urethra (Fig. 1A–D,F). Surrounding the mucosa of the pelvic urethra are the ducts of the accessory sex glands: ventral and proximal to the pelvic urethra are the ventral prostatic ducts (Figs 2D and 3D); dorsal are the coagulating gland ducts, preceding the opening of the ampullary duct. The ampullary duct received the ducts of the seminal vesicle, of the ductus deferens gland and the papilla of the ductus deferens, later to open up in the dorsal surface of the pelvic urethra. The dorsolateral prostatic ducts drained into the dorsal surface of the pelvic urethra simultaneously to the ampullary duct (Fig. 3D). The bulbourethral gland drained directly into the penile bulb that contains the urethral diverticle (Fig. 4A).
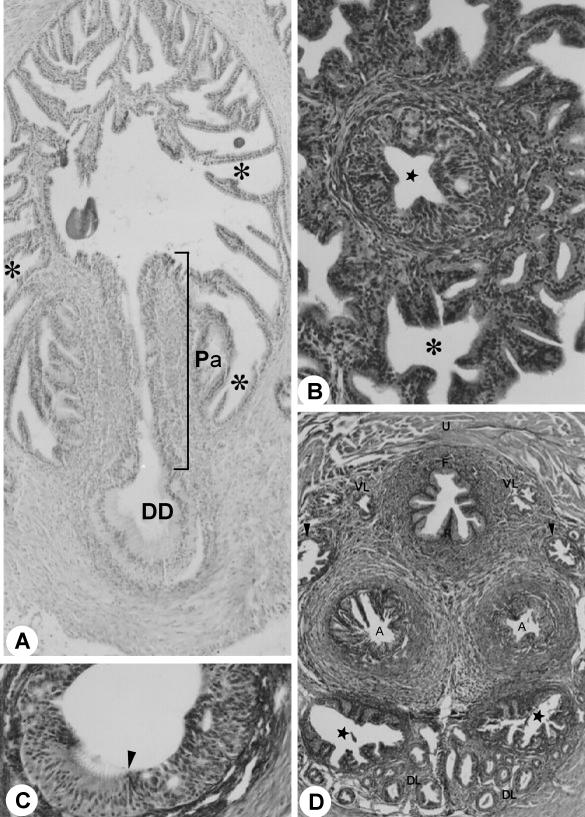
Fig. 3.
(A) Ducts of the ductus deferens gland (asterisk), Pa. HE stain. ×733. DD, ductus deferens. (B) Cross-section through the ductus deferens papilla. Star, papilla; asterisk, excurrent ducts of the ductus deferens gland. MlT stain. ×430. (C) Cross-section of the transition (arrow) of the epithelium of the ductus deferens papilla. MlT stain. ×200. (D) Cross-section through of the roof and of the floor of the pelvic urethra. Arrowhead, duct of the coagulating gland; star, duct of the seminal vesicle. MlT stain. ×214. Compare with the disposition of the ducts of the sex accessory gland in Fig. 2(D). For abbreviations, see text.
The seminal vesicle duct comprises a simple cylindrical epithelium, with nucleus located in the basal third of the cell with nucleolus evident and with a granular cytoplasm. Delicate folds of the mucosa and a single smooth muscle cell layer (myoepithelium) were observed involving the entirety of the duct. The seminal vesicle duct was located dorsally with respect to the ductus deferens en route to its opening into the ampullary duct. Next to the roof of the pelvic urethra, the seminal vesicle duct was connected to several dorsolateral prostatic ducts and fibromuscular tissue (Fig. 3D).
The coagulating gland duct is located laterally and ventrally to the ductus deferens, opening craniolaterally to the ostium of the ampullary duct in the roof of the pelvic urethra (Fig. 3D). The coagulating gland duct presented a simple cylindrical epithelium (Fig. 4C).
The ventral prostatic ducts presented simple cylindrical epithelium (Fig. 4E) with folds in the mucosa. The dorsal prostatic ducts opened in the roof of the pelvic urethra, connected by smooth muscle fibres (Fig. 4B). The prostatic ducts, close to the urethra, presented a thick layer of smooth muscle cells, guiding the ducts until the urethral mucosa, forming paths between urethral muscles.
The ductus deferens gland ducts presented simple cylindrical epithelium, distributed in radial form in the muscle layer of the ductus deferens toward the ampullary duct to drain the glandular secretion (Fig. 3B).
The distal (terminal) portion of the ductus deferens was characterized by the transition of the epithelium from a pseudostratified type with stereocilia to a stratified cylindrical type (Fig. 3A,C). Next to the pelvic urethra we observed the formation of the ductus deferens papilla opening the ampullary duct. This duct resulted from confluence of the ducts of the ductus deferens gland and the origin of the papilla of the ductus deferens. The papilla presented a star-shaped format, consisting of the stratified cylindrical epithelium (Fig. 3B). At its origin, next to the roof of the pelvic urethra, the ampullary duct appeared to be flanked by the dorsal prostate, seminal vesicle and coagulating gland ducts. The ampullary duct opened in the roof of the pelvic urethra (Fig. 4A).
The bulbourethral gland duct was lined with epithelium that varied from cylindrical to paving-stone shaped. The ducts congregated into only one duct, accompanied by glandular tissue, surrounded by a single smooth-muscle-cell layer (myoepithelium), muscular fibres, adipose tissue and a serosa layer. The duct extended close to the bulb of the penis and opened into the wall of the diverticle of the urethra (Fig. 6B).
Discussion
Dellmann (1993) considered three distinct portions in the urethra: pelvic, from the neck of the urinary bladder to the penile bulb; bulbar contained in the penile bulb; and penile, from the bulbous extremity to the external ostium of the urethra. Getty (1986) and Dyce et al. (1997) described two portions of the male urethra in domestic animals: pelvic, from the internal ostium of the urethra to the seminal colliculus; and penile, from the seminal colliculus to the external ostium of the urethra. According to Nakano & Muto (1989), the urethra of various mammals including man is divided into three parts: prostatic, membranous and spongy (penile). The prostatic urethra is lined with the transitional epithelium, while the membranous and spongy parts are lined with stratified (or pseudostratified) columnar epithelium. According to Carrol & Dixon (1992), anatomically the human male urethra can be divided into posterior and anterior. The posterior urethra is composed of the membranous and prostatic segments. The anterior urethra is composed of the penile urethra with its bulbar and pendulous segments. Williams et al. (1989) decsribe four regional parts in the human male urethra: preprostatic, prostatic, membranous and spongiose. In the rat and mouse, according to Hayes (1965), the urethra presents two portions: pelvic and penile. The pelvic urethra initiates in the internal ostium of the urethra and finishes in the penile bulb. The penile bulb contains a large urethral diverticle, an expansion of the penile urethra. The penile urethra initiates in the penile bulb and extends to the external ostium of the urethra. In the gerbil, the urethra was divided into pelvic and penile, similar to that seen in the rat, mouse and domestic animals.
The penile bulb of the gerbil shelters the urethral diverticle that receives the duct of the bulbourethral gland. The diverticle was situated between the pelvic and penile urethra, matching the point of urethral transition, the isthmus. The topographical anatomical position of the urethral diverticle in the gerbil is comparable to that of the rat and the mouse (Hayes, 1965; Çiner et al. 1996) and with the suburethral diverticle in the sow and the cow. In these animals the urethra forms a small pouch immediately before entering the vagina (Çiner et al. 1996). Among domestic mammals, the male urethra of small ruminants extends beyond the tip of the penis, forming the urethral process (processus urethrae) (Ghoshal & Bal, 1976), and is not a homologue of the urethral diverticle in the gerbil, rat or mouse. Goats, sheep, cattle and swine present a urethral recess projecting dorsocaudally in the region of the junction between the pelvic and penile urethra. The urethral recess constitutes the urethral diverticle that, like that in the Muridae, receives the duct of the bulbourethral gland (Garrett, 1987).
The urethral diverticle of the gerbil is heart-shaped, presenting bilateral symmetry. In accord with Çiner et al. (1996), the bifid organization of the diverticle, also heart-shaped, can be considered a remnant from an original paired system, which is fused incompletely in the Muridae.
Next, the pelvic urethra of the gerbil presents its transition epithelium origin, changing to stratified (or pseudostratified) cylindrical, being similar therefore to those of man (Leeson & Leeson, 1977) and mouse (Nakano & Muto, 1989). Already, the penile urethra presents a lesser diameter than the pelvic urethra. The stratified cylindrical epithelium and rich propria lamina in the elastic, collagenous and smooth muscle fibres determine the folds in the mucosa (Leeson & Leeson, 1977; Nakano & Muto, 1989; Dellmann, 1993).
Throughout the extension of the male gerbil urethra, in the mucosa, urethral glands were observed, related to those of female rodents (Shehata, 1974), but rarely identified in humans (Newman & Hicks, 1981). The appearance of the female urethral glands only differs from the male in the openings of the ducts.
The glandular portions of the seminal vesicle are constituted by a simple cylindrical epithelium. The duct is located dorsally to the ductus deferens and its opening occurs in the ampullary duct, as in the albino rat (Hebel & Stromberg, 1976). In the gerbil, there is no formation of the ejaculatory duct as in primates, and in this way the gerbil is similar to the chinchilla (Martinez et al. 1995). The duct of the coagulating gland opens independently in the roof of the urethra, as occurs in the albino rat (Jesik et al. 1992). In the gerbil, a larger number of ventral ducts drain the dorsolateral lobe of the prostate. The ducts drain independently in the pelvic urethra. According to Jesik et al. (1992), the ventral lobes are drained by four or five ducts that originate in the central portion of the lobe and follow a straightforward course to the ventrolateral muscle layers of the urethra wall. Small ducts are occasionally observed that drain the acini and enter the main channels, which in turn empty into the urethra. In the lateral lobe, each duct drains only three or four acini, and the acini and their ducts are wrapped in connective tissue. There are seven or eight ducts draining each lateral lobe. The portions of the lateral lobe closest to ventral lobe area are drained by two ducts that enter the urethral lumen ventrolaterally. Four ducts that enter the urethral wall medially drain the middle portion of the lateral lobes. The most anterior parts of the lateral lobes are drained by one or two ducts that enter the dorsal urethra. The dorsal lobes are each drained by 10–14 long and slender ducts that enter the dorsal roof of the urethra. In the gerbil, it was not possible to quantify the number of ducts. However, in the albino rat it was shown that the biggest prostatic lobe (dorsolateral) possesses the greatest number of ducts. The heights of the epithelium in the ventral and dorsolateral prostatic lobes and in the ductus deferens gland do not present differences among themselves.
The ducts of the ductus deferens glands form diverticles, surrounding the papilla of the ductus deferens, constituting the ampullary duct, similar to those described in the albino rat (Hebel & Stromberg, 1976). They are presented firstly as next to the muscular layer of the ductus deferens and, subsequently, constitute the ampullary duct.
The organization of the parenchyma, stroma and distribution of the ducts of the bulbourethral gland of the gerbil was shown to be similar to that in the albino rat (Hebel & Stromberg, 1976) and man (Junqueira & Carneiro, 1995).
The ducts of the seminal vesicle, the ductus deferens gland and ductus deferens, drain their contents independently through the ampullary duct that opens into the pelvic urethra. The prostatic ducts and both coagulating and bulbourethral glands drain their contents independently into the pelvic urethra. It has been concluded that the structures of the urethra and of the ducts are similar to those of the albino rat (Hebel & Stromberg, 1976), the mouse (Greene, 1966) and the golden hamster (Silva et al. 1995). However, there are variations in the disposition of the opening of the ducts of the sex accessory glands toward the interior of the urethra.
| A | ampullary duct |
| B | bulbospongiosus muscle |
| BG | bulbourethral gland |
| C | corpus cavernosum |
| CG | coagulating gland/duct of the coagulating gland |
| D | duct of the bulbourethral gland |
| DD | ductus deferens |
| DL | dorsal prostatic lobe/dorsal prostatic duct |
| E | corpus spongiosum |
| F | floor of the urethra |
| GDD | ductus deferens gland |
| L | lateral part of the bulboespongiosus muscle |
| M | medial part of the bulboespongiosus muscle |
| P | penis |
| Pa | papilla of the ductus deferens |
| PEU | penile urethra |
| PU | pelvic urethra |
| R | roof of the urethra |
| SV | seminal vesicle/duct of the seminal vesicle |
| T | testis |
| U | urethral muscle |
| UB | urinary bladder |
| UD | urethral diverticle |
| UG | urethral gland |
| VL | ventral prostatic lobe/ventral prostatic duct |
Acknowledgments
We gratefully acknowledge the collaboration of Professor Francisco Eduardo Martinez of the University of the State of São Paulo, Botucatu, as well as the support given by FAPESP (98/11142–9).
References
- Carrol PR, Dixon CM. Surgical anatomy of the male and female urethra. Urologic Clinics North Am. 1992;19:339–346. [PubMed] [Google Scholar]
- Çiner M, Van Vorstenbosch CJAHV, Dijkstra G. Penile bulb and its relationship with the pelvic urethra and the penile urethra in the rat: Light and scanning electron microscopical observations. Anat. Rec. 1996;244:452–469. doi: 10.1002/(SICI)1097-0185(199604)244:4<452::AID-AR4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Dellmann HD. Textbook of Veterinary Histology. 4th edn. Philadelphia: Lea & Febiger; 1993. [Google Scholar]
- Dyce KM, Sack WO, Wensing CLG. Tratado De Anatomia Veterinária. 2nd edn. Rio de Janeiro: Guanabara Koogan; 1997. [Google Scholar]
- Fulker MJ, Cooper EH, Tanaka T. Proliferation and ultrastructure of papillary transitional. Cell of the human bladder. Cancer. 1971;27:71. doi: 10.1002/1097-0142(197101)27:1<71::aid-cncr2820270112>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Garrett PD. Urethral recess in male goats, sheep, cattle, and swine. JAVMA. 1987;191:689–691. [PubMed] [Google Scholar]
- Getty R. Anatomia Dos Animais Domésticos. 2nd edn. Rio de Janeiro: Guanabara Koogan; 1986. [Google Scholar]
- Ghoshal NG, Bal HS. Histomorphology of the urethral process of the goat (Capra hircus) Acta Anat. 1976;94:567–573. [PubMed] [Google Scholar]
- Greene EL. Biology of the Laboratory Mouse. 2nd edn. New York: McGraw-Hill Co; 1966. [Google Scholar]
- Hayakawa M. The clinical and cytological studies on urothelial tumours of the upper urinary tract. Jpn. J. Urol. 1979;69:1439. [Google Scholar]
- Hayes KJ. The so-called levator ani of the rat. Acta Endocrinol. 1965;48:337–347. doi: 10.1530/acta.0.0480337. [DOI] [PubMed] [Google Scholar]
- Hebel R, Stromberg MW. Anatomy of the Laboratory Rat. Baltimore: Williams & Wilkins Co; 1976. [Google Scholar]
- Hummel KP, Richardson FL, Fekete E. Anatomy. In: Greene EL, editor. Biology of the Laboratory Mouse. New York: McGraw-Hill; 1966. pp. 247–307. [Google Scholar]
- Jesik CJ, Holland JM, Lee C. An anatomic and histologic study of the rat prostate. Prostate. 1992;3:81–97. doi: 10.1002/pros.2990030111. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Carneiro J. Histologia Básica. 8th edn. Rio de Janeiro: Guanabara Koogan; 1995. [Google Scholar]
- Kanagasuntheram R, Anandarajara S. Development of the terminal urethra and prepuce in the dog. J. Anat. 1960;94:124–129. [PMC free article] [PubMed] [Google Scholar]
- Kjaer TB, Carlson SD, Nilsson T, Madsen PO. Scanning electron microscopy of the normal and malignant human urothelium. Urology. 1976;8:59. doi: 10.1016/0090-4295(76)90058-3. [DOI] [PubMed] [Google Scholar]
- Kumagai I. A scanning electron microscopic study on the urothelium. I. Ultrastructure of the urothelial surface of the bladder. Jpn. J. Urol. 1975;66:131. doi: 10.5980/jpnjurol1928.66.3_131. [DOI] [PubMed] [Google Scholar]
- Leeson RC, Leeson TS. Histologia. 3rd edn. Rio de Janeiro: Interamericana; 1977. [Google Scholar]
- Martinez FE, Martinez M, Garcia PJ, Cagnon VHA, Gralhoz G, Dos Santos CXC. Morfologia da vesícula seminal da chinchila (Chinchilla laniger) Rev. Ciênc. Bioméd. 1995;16:17–23. [Google Scholar]
- Nakano T, Muto H. Scanning electron microscopic observation on the distal part of the male urethra of the mouse. Z. Mikrosk. Anat. Forsch. Leip. 1989;103:28–35. [PubMed] [Google Scholar]
- Nelson CE, Croft WA, Nilsson T. Characterization of different regions of the normal human urinary bladder by means of scanning electron microscopy. Scand. J. Urol. Nephrol. 1979;1:23. doi: 10.3109/00365597909179996. [DOI] [PubMed] [Google Scholar]
- Newman J, Hicks RM. Detection of the neoplastic and preneoplastic urothelia by combined scanning and transmission electron microscopy of the urinary surface of human and rat bladders. Histopathology. 1977;1:125. doi: 10.1111/j.1365-2559.1977.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Newman J, Hicks RM. Surface ultrastructure of the epithelia lining the normal human lower urinary tract. Br. J. Exp. Pathol. 1981;62:232–251. [PMC free article] [PubMed] [Google Scholar]
- Quayle AJ, Pudney J, Muñoz DE, Anderson DJ. Characterization of the T limphocytes antigen-presenting cells in the murine male urethra. Biol. Reprod. 1994;51:809–820. doi: 10.1095/biolreprod51.5.809. [DOI] [PubMed] [Google Scholar]
- Shehata R. Urethral glands in the wall of the female urethra of rats, mice and closely related rodents. Acta Anat. 1974;90:381–387. doi: 10.1159/000144345. [DOI] [PubMed] [Google Scholar]
- Silva TP, Orsi AM, Goes ERC. Características morfológicas do ducto deferente do hamster dourado (Mesocricetus auratus, W) Rev. Ciênc. Bioméd. 1995;16:37–45. [Google Scholar]
- Sprando RL. Perfusion of the rat testis through the heart using heparin. In: Russell LD, Ettlin RA, Hikkim APS, Clegg ED, editors. Histological and Histopathological Evaluation of the Testis. Clearwater: Cache River; 1990. pp. 277–280. [Google Scholar]
- Tannenbaum M, Tannenbaum S, Carter HW. SEM, BEL and TEM ultrastructural characteristics of the normal preneoplastic and neoplastic human transitional epithelial. Scanning Electron Microscop. 1978;2:949. [Google Scholar]
- Williams PL, Warwick R, Dyson M, Bannister LH. Gray's Anatomy. 37th edn. Edinburgh: Churchill Livingstone; 1989. [Google Scholar]