Abstract
Matrix metalloproteinases (MMPs) have been implicated in physiological cartilage matrix remodelling as well as in pathological and invasive extracellular matrix remodelling of tissue. Age-related changes in the gene expression patterns of MMPs in mandibular condylar cartilages (MCCs) were analysed. We examined the gene expression patterns of Mmp-8 and -13 and their substrates, Col1a1, Col2a1 and Col10a1, in MCC of growing and ageing rats. Temporomandibular joints of male Wistar rats aged 4, 8, 16 and 32 weeks were subjected to in situ hybridization analysis. Histologically, MMCs showed characteristics of growth plate cartilage at ages 4 and 8 weeks, and more closely resembled articular cartilage thereafter. Mmp-8 was expressed in the cells in all cartilaginous cell layers at ages 4 and 8 weeks, and then was localized only in the mature cells at ages 16 and 32 weeks. Whereas Mmp-13 expression was limited to the lowermost hypertrophic chondrocytes in the growth stage, mature chondrocytes instead of hypertrophic chondrocytes expressed Mmp-13 in adult non-hypertrophic MCC. Because Mmp-8 and -13 expression overlapped with Col2a1 and Col10a1, chondrocytes could play a pivotal role in degradation as well as production of the cartilaginous matrix in MCC.
Keywords: collagenase, in situ hybridization, MMP-8, MMP-13, type II collagen, type X collagen
Introduction
The mandibular condylar cartilage (MCC) has been known both as a major growth site of the mandible and as an articular cartilage. It plays an important role in determining facial morphology and in orofacial complex functions such as mastication and speech. During the growth period, MCC manifests characteristics of both growth plate cartilage and articular cartilage; however, it functions only as an articular cartilage at the end of growth (Silbermann et al. 1987). During the growth period, MCC consists of a five-layered growth plate cartilage comprised of (starting from the articular surface) a fibrous layer, a proliferative cell layer, a transitional cell layer, a mature cell layer and a hypertrophic cell layer (Luder et al. 1988; Mizoguchi et al. 1996). At the end of the growth period, MCC remains on the articular surface of the mandibular condyle as an articular cartilage (Takahashi et al. 1996). The layered composition of MCC becomes similar to that of the articular cartilage of the long bone at this time, basically consisting of three layers (fibrous, proliferative and mature), except that the fibrous layer is absent on the articular surface of the long bone.
Many studies have been performed to verify the composition of the extracellular matrix (ECM) of MCC relating to growth and ageing (Takahashi et al. 1996; Ohashi et al. 1997). It has long been known that collagens, proteoglycans and other non-collagenous proteins comprise the ECM of MCC (Buckwalter & Rosenberg, 1988; Luder et al. 1988; Silbermann & von der Mark, 1990; Ishibashi et al. 1996), and the majority of the cartilage of ECM has been considered to consist of collagens. Both type II and type X collagens are localized in the cartilaginous cell layers beyond the mature and hypertrophic cell layers as markers for chondrocytes and within hypertrophic chondrocyte, respectively (Silbermann & von der Mark, 1990; Salo et al. 1996). Chondroitin sulphates are distributed throughout all the layers of MCC, and keratan sulphate is localized in the cartilaginous layers (Takahashi et al. 1996; Mizoguchi et al. 1997). Type I collagen and fibronectin have been localized in the non-cartilaginous cell layers of MCC in growing animals (Ben Ami et al. 1991; Ishibashi et al. 1996; Mizoguchi et al. 1997). Thus, many studies of MCC have attempted to clarify the distribution of collagens, proteoglycans and glucosamine glycans (GAGs) during the growth and ageing process, but the age-related changes in the gene expression patterns of collagens are unknown.
Cellular migration and tissue remodelling have been known to require the degradation of ECM through the action of a family of zinc-dependent endopeptidases, the matrix metalloproteinases (MMPs). These enzymes are concerned with many physiological processes and pathogeneses such as fetal development (Chin & Werb, 1997; Werb & Chin, 1998), the wound healing process (Planus et al. 1999), angiogenesis (Vu et al. 1998), cancer cell invasion (Kleiner & Stetler-Stevenson, 1999) and cartilaginous diseases such as osteoarthritis (Cawston, 1998; Walter et al. 1998). Although chondroclasts expressing proteolytic lysozomal enzymes such as cathepsin K degrade cartilaginous matrix at the osteochondral junction (Yamaza et al. 1998; Uusitalo et al. 2000), it has been also known that MMPs are key enzymes of ECM metabolism (Vu et al. 1998; Armstrong et al. 2002; Wu et al. 2002). MMPs have been classified into five subgroups: collagenases (MMP-1, -8 and -13), gelatinases (MMP-2 and -9), stromelysins (MMP-3, -10 and -11), membrane type (MT) MMPs (MT-1 to -6 MMPs) and others (reviewed by Cawston, 1998; Nagase & Woessner, 1999). Major MMPs that degrade collagens, which are the major macromolecules in the cartilage ECM, are collagenases and gelatinases (Shingleton et al. 1996; Yocum et al. 1999). Although the functions of MMPs in the pathogenetic processes described above have been known, their expression patterns in normal tissues including cartilage are largely unknown.
In the present study, we examined the age-related changes in the gene expression patterns of collagenases in relation to the expression of their substrates in MCC of rats. We hypothesized that, during the growth and ageing process of MCC, the gene expressions of the major collagenases, MMP-8 and -13, are correlated to the gene expression of their major substrates, type I, II and X collagens.
Materials and methods
Animals and tissue preparation
Three male Wistar rats at 4, 8, 16 and 32 weeks of age were used in the present study. Animals were anaesthetized with 10 mg kg−1 of pentobarbital, and perfused by 4% paraformaldehyde and 0.5% glutaraldehyde in 0.1 m phosphate-buffered saline (PBS; pH 7.4) from the ascending aorta for 30 min at the rate of 3 mL min−1. After the perfusion, the temporomandibular joints (TMJs) were dissected and further fixed in the same fixatives overnight at 4 °C. All the specimens were thoroughly rinsed and decalcified in 10% ethylene diamine tetra acetic acid (EDTA) in 0.01 m PBS (pH 7.4) for 1–8 weeks at 4 °C. Specimens were handled in RNase-free conditions. Specimens were embedded in paraffin after being dehydrated through a graded series of ethanol baths. Eight-micrometre-thick sections were cut for in situ hybridization (ISH) analysis and haematoxylin and eosin (H&E) staining.
In addition, limb buds of embryonic day 16 Wistar rats were used to prepare a cDNA source. After the rat embryos were dissected out, fore- and hind-limb buds were isolated, and then total RNA was extracted using a total RNA isolation kit (Qiagen, Hilden, Germany), following the manufacturer's instructions. Obtained total RNA was reverse transcribed into cDNA using a SuperScript II reverse transcription kit (Invitrogen, Carlsbad, CA, USA) by following the manufacturer's instructions for preparation of riboprobes.
Histological examination
Eight-micrometre-thick sagittal serial sections of the TMJ from 4-, 8-, 16- and 32-week-old rats were deparaffinized, rehydrated in a graded series of ethanol baths, then stained with H&E for light microscopic examination.
Preparation of riboprobes
Mmp-8, Mmp-13, Col1a1 and Col2a1 riboprobes were used as previously described by Sasano et al. (2002). A 570-bp fragment of rat Col10a1 cDNA (572–1141 bp of GenBank accession No. AJ131848, 5′-ACAACAGGCAGCAGCACTA-3′ for sense and 5′-AGGATGGGACGACAGGAG-3′ for antisense primers), which was obtained by reverse transcription-polymerase chain reaction, was subcloned into pCRIITOPO vector plasmids (Invitrogen). Forty cycles of PCR amplification were carried out using a RoboCycler (Stratagene, La Jolla, CA, USA) under the following conditions: denaturation at 95 °C for 70 s, annealing at 55 °C for 70 s, and extension at 72 °C for 2 min. The nucleic acid sequence of Col10a1 cDNA was analysed by TaKaRa (Osaka, Japan). To create digoxygenin (DIG)-labelled riboprobes, the plasmids were linearized by restriction enzymes (EcoRV for antisense and SpeI for sense), and were transcribed by Sp6 RNA polymerases (Stratagene) for antisense, and T7 RNA polymerases (Stratagene) for sense riboprobes.
The protocol for ISH was as described in our previous report (Sasano et al. 2002). Briefly, sections were deparaffinized, rehydrated in a graded series of ethanol baths, and then immersed in 0.2 n HCl for 20 min. After incubation in proteinase K (20 g mL−1; Roche Diagnostics, Indianapolis, IN, USA) at 37 °C for 30 min, sections were dehydrated in ethanol. Air-dried sections were hybridized using approximately 400 ng mL−1 of antisense or sense riboprobes in the hybridization buffer at 45 °C for 16 h. After washing twice with concentrated SSC and treating RNase A (Sigma, St Louis, MO, USA) 20 µg mL−1 for 20 min at 37 °C, the sections were incubated overnight at 4 °C with anti-DIG alkaline phosphate antibody (Roche Diagnostics) diluted 1 : 500 with PBS and 0.5% bovine serum albumin. After washing with Tris-buffered saline, the sections were treated by 4-nitro blue tetrazolium chloride (500 µg mL−1) and 5-bromo-4-chloro-3-indolyl-phosphate (187.5 µg mL−1) (Roche Diagnostics) for visualization. The sections were counterstained by methyl green, and mounted in mounting media (Vector Laboratories, Burlingame, CA, USA).
Results
Histological observation
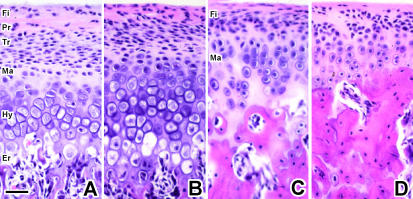
MCC showed characteristics of growth plate cartilage at 4 and 8 weeks of age, but then more closely resembled articular cartilage at 16 weeks of age and thereafter (Fig. 1). When MCC was similar to growth plate cartilage, it consisted of five layers: (1) a fibrous layer with fibroblasts embedded in the fibrous connective tissue; (2) a proliferative cell layer with undifferentiated and proliferous polygonal-shaped cells; (3) a transitional cell layer with flattened cells, without lipid drops in cytozol; (4) a mature cell layer with ovoid-shaped chondrocytes, which were differentiated and had cell polarity; and (5) a hypertrophic cell layer with enlarged cells with disorganized cytozolic structures (Fig. 1A,B). An erosion zone was observed beyond the hypertrophic cell layers in MCC at age 4 and 8 weeks (Fig. 1A,B).
Fig. 1.
Sagittal sections of MCC and tibial articular cartilage stained by H&E. A, B, C and D are MCC at 4, 8, 16 and 32 weeks of age, respectively. Fi: fibrous layer, Pr: proliferative cell layer, Tr: transitional cell layer, Ma: mature cell layer, Hy: hypertrophic cell layer, Er: erosion zone, Re: resting cell layer. Scale bar = 100 µm; original magnification: ×40.
By contrast, MCC at 16 and 32 weeks of age appeared to be articular cartilage lacking a hypertrophic cell layer (Fig. 1C,D). The uppermost layer of aged MCC was fibrous with elongated fibroblasts embedded in the fibrous connective tissue; the second layer was proliferative, with small undifferentiated cells; and the lower layer was mature, with well-differentiated chondrocytes.
Gene expression patterns (Figs. 2 and 3)
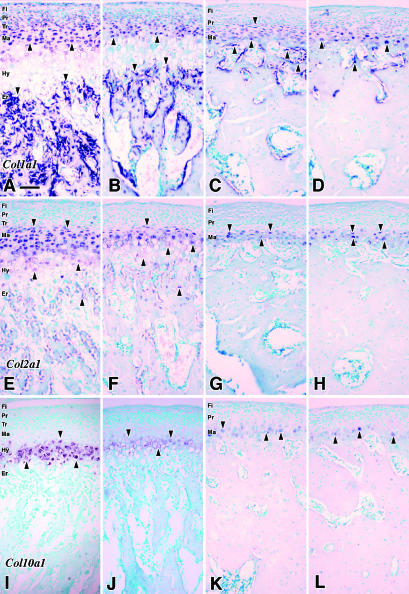
Fig. 2.
The results of in situ hybridization (ISH) in sagittal sections of MCC. The expression patterns of Col1a1 mRNA are shown in A–D. E–H show the signals of Col2a1 mRNA, and I–L show the expression patterns of Col10a1 mRNA. A, E and I are 4 weeks of age; B, F and J are 8 weeks of age MCC; C, G and K are 16 weeks of age; D, H and L are 32 weeks of age. The arrows indicate the positive ISH signals. Fi: fibrous layer, Pr: proliferative cell layer, Tr: transitional cell layer, Ma: mature cell layer, Hy: hypertrophic cell layer, Er: erosion zone. Scale bar = 100 µm; original magnification: ×20.
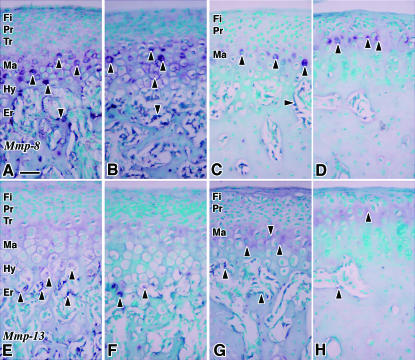
Fig. 3.
The expression patterns of Mmp-8 mRNA (A–D) and Mmp-13 mRNA (E–H) in MCC. A and E are 4 weeks of age; B and F are 8 weeks of age; C and G are 16 weeks of age; D and H are 32 weeks of age. The arrows indicate the positive signals of in situ hybridization. Fi: fibrous layer, Pr: proliferative cell layer, Tr: transitional cell layer, Ma: mature cell layer, Hy: hypertrophic cell layer, Er: erosion zone. Scale bar = 100 µm; original magnification: ×40.
Collagens
Positive hybridization signals for Col1a1 were observed in mature chondrocytes throughout the experimental period (Fig. 2A,D). At 4 and 8 weeks of age, cells in all of the layers except for the hypertrophic cell layer showed positive signals (Fig. 2A,B), whereas the signals were quite weak in the fibrous layer, and were restricted to proliferative and mature cells at 16 and 32 weeks of age (Fig. 2C,D). Osteoblasts lying on the trabecular bone surface beyond the erosion zone presented strong positive signals for Col1a1 throughout the experiments.
Throughout the experimental period, strong hybridization signals for Col2a1 were observed in the cells of the mature cell layer of MCC (Fig. 2E–H). Relatively weak signals, compared with the upper layers, were observed in the hypertrophic cell layer at 4 and 8 weeks of age (Fig. 2E,F), and the signals disappeared at 16 and 32 weeks of age (Fig. 2G,H). Osteoblasts, lying on the trabecular bone beyond the erosion zone, presented weak positive signals at 4 and 8 weeks of age; however, they disappeared at older ages.
Positive hybridization signals for Col10a1 were restricted to cells in the hypertrophic cell layers at 4 and 8 weeks of age (Fig. 2I,J). They were then found in cells in the mature cell layer at 16 and 32 weeks of age (Fig. 2K,L). In the older age samples, strength of the positive signals fluctuated among the cells (Fig. 2K,L). No positive hybridization signals for Col10a1 were observed in any other types of cells.
MMPs
Positive hybridization signals for Mmp-8 were observed in cells throughout the cartilaginous cell layers, in osteoblasts lying on the trabecular bone surface and in bone marrow cells at 4 and 8 weeks of age (Fig. 3A,B). At 16 weeks of age, cells in the top of the mature cell layer showed relatively stronger signals than those seen in the other parts of this layer (Fig. 3C). Osteocytes in the mandibular condyle also presented hybridization signals for Mmp-8 (Fig. 3C). Only cells in the top of the mature cell layer showed positive signals at 32 weeks of age; however, the signal strength fluctuated among the cells (Fig. 3D). The localization of positive signals was observed in all cell layers at 4 weeks of age, and was progressively restricted to the cells of the mature cell layer as the animals aged.
Cells in the last row of the hypertrophic cell layers presented positive hybridization signals for Mmp-13 at 4 and 8 weeks of age, and relatively weak signals were also observed in the hypertrophic cells (Fig. 3E,F). Weak positive signals were observed in cells in the mature cell layer at 16 and 32 weeks of age (Fig. 3G,H).
Negative control experiments (Fig. 4)
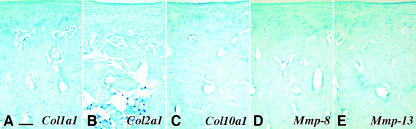
Fig. 4.
The results of in situ hybridization using sense DIG-labelled riboprobes for negative control experiments from 16-week-old MCC. No positive signal of Col1a1 sense riboprobe was observed in A; Col2a1 sense riboprobe in B; Col10a1 sense riboprobe in C; Mmp-8 sense riboprobe in D; Mmp-13 sense riboprobe in E. Scale bar = 200 µm; original magnification: ×20.
The results of the negative control experiments are indicated in Fig. 4. No hybridization signals were observed in any sections hybridized with any sense riboprobes. All of the samples were subjected to ISH using sense DIG-labelled riboprobes; however, only the measurements for MCC at 16 weeks of age are indicated in the figure.
Discussion
Previous studies have reported that type I collagen is localized in the upper non-cartilaginous cell layers of MCC (Mizoguchi et al. 1990, 1996; Silbermann & von der Mark, 1990), and type II collagen is localized in the cartilaginous cell layers of MCC in growing rats or mice (Luder et al. 1988; Mizoguchi et al. 1990, 1996). Immunoreactivity for type X collagen antibody is mainly detected in the hypertrophic cell layers (Salo et al. 1996). The gene expression patterns of collagens during the growth period demonstrated in the present study coincided with the patterns of those demonstrated by immunohistochemistry.
MMP-8 is generally expressed in neutrophils (Lazarus et al. 1968a, b), and our previous report (Sasano et al. 2002) demonstrated that Mmp-8 is expressed in the mature chondrocytes in embryonic long bone cartilages. The present results support the previous data and add that Mmp-8 is expressed not only in growth plate cartilages, but also in mature chondrocytes in MCC during the growth period and ageing process. By contrast, MMP-13 is localized in the lowermost hypertrophic chondrocytes of growth plate cartilage, as described in previous reports (Gack et al. 1995; Tuckermann et al. 2000). The expression pattern of Mmp-13 mRNA observed during the growth period in the present study supports those previous reports. Thus, MMP-8 and MMP-13 expressed during the growing period could share the functions of remodelling ECM during the differentiation of chondrocytes and degrading cartilaginous ECM accompanying the endochondral bone formation, respectively.
After the end of growth, MCC functions as an articular cartilage in the temporomandibular joint and the chondrocytes contribute to the metabolism of cartilage tissue. Ohashi et al. (1997) reported changes in localization of type I, II and X collagen in rat MCC during the ageing process, and mentioned that the distribution of type X collagen expanded over the mature cell layer in the 4-month-old rat; however, gene expression was limited to small numbers of cells in the mature cell layer as shown in the present study. As the gene expression patterns in the growing period covered the protein distribution, this discrepancy in the ageing period could indicate that small numbers of mature chondrocytes contribute to the expression of ECM molecules rather than indicating the difference in the sensitivity of two distinct methods. By contrast, whereas Mmp-8 is expressed in mature chondrocytes in the adult MCC, the expression of Mmp-13 disappeared during the ageing period in which the endochondral bone formation was completed, based on the results of the present study. Similar to the expression of collagen genes, the cells in the mature cell layer expressed the Mmp-8 gene. Therefore, the limited numbers of cells in the mature cell layer of MCC contribute to maintaining the metabolism of the cartilaginous matrix in MCC.
In conclusion, chondrocytes in MCC express both ECM genes and MMP genes during the growing period and the ageing process in rats. MMP-8 may contribute to ECM remodelling throughout the life span of cartilaginous tissues, whereas MMP-13 plays a role in cartilage degradation only during the growth period. In the present study, we demonstrated only the mRNA expression patterns of MMPs and collagens, and additional immunohistochemical or zymographical investigations will be needed to expand further our understanding of the functions of MMPs in cartilage metabolism.
Acknowledgments
We are grateful to Drs Koji Kindaichi and Hirotoshi Akita, Division of Oral Molecular Biology, Department of Oral Biology, Tohoku University Graduate School of Dentistry, for their valuable advice. We also thank Mr Masami Eguchi, Mr Yasuto Mikami and Mr Toshihiro Onodera for technical assistance. This research was partially supported by Grants-in-Aid (#11771308 and #12557180) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
References
- Armstrong AL, Barrach HJ, Ehrlich MG. Identification of the metalloproteinase stromelysin in the physis. J. Orthop. Res. 2002;20:289–294. doi: 10.1016/S0736-0266(01)00120-6. [DOI] [PubMed] [Google Scholar]
- Ben Ami Y, von der Mark K, Franzen A, De Bernard B, Lunazzi GC, Silbermann M. Immunohistochemical studies of the extracellular matrix in the condylar cartilage of the human fetal mandible: collagens and noncollagenous proteins. Am. J. Anat. 1991;190:157–166. doi: 10.1002/aja.1001900205. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Rosenberg LC. Electron microscopic studies of cartilage proteoglycans. Electron Microsc. Rev. 1988;1:87–112. doi: 10.1016/s0892-0354(98)90007-7. [DOI] [PubMed] [Google Scholar]
- Cawston T. Matrix metalloproteinases and TIMPs: properties and implications for the rheumatic diseases. Mol. Med. Today. 1998;4:130–137. doi: 10.1016/s1357-4310(97)01192-1. [DOI] [PubMed] [Google Scholar]
- Chin JR, Werb Z. Matrix metalloproteinases regulate morphogenesis, migration and remodeling of epithelium, tongue skeletal muscle and cartilage in the mandibular arch. Development. 1997;124:1519–1530. doi: 10.1242/dev.124.8.1519. [DOI] [PubMed] [Google Scholar]
- Gack S, Vallon R, Schmidt J, Grigoriadis A, Tuckermann J, Schenkel J, et al. Expression of interstitial collagenase during skeletal development of the mouse is restricted to osteoblast-like cells and hypertrophic chondrocytes. Cell Growth Differ. 1995;6:759–767. [PubMed] [Google Scholar]
- Ishibashi H, Takenoshita Y, Ishibashi K, Oka M. Expression of extracellular matrix in human mandibular condyle. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996;81:402–414. doi: 10.1016/s1079-2104(96)80015-1. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother. Pharmacol. 1999;43:S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- Lazarus GS, Brown RS, Daniels JR, Fullmer HM. Human granulocyte collagenase. Science. 1968a;159:1483–1485. doi: 10.1126/science.159.3822.1483. [DOI] [PubMed] [Google Scholar]
- Lazarus GS, Daniels JR, Brown RS, Bladen HA, Fullmer HM. Degradation of collagen by a human granulocyte collagenolytic system. J. Clin. Invest. 1968b;47:2622–2629. doi: 10.1172/JCI105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luder HU, Leblond CP, von der Mark K. Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Am. J. Anat. 1988;182:197–214. doi: 10.1002/aja.1001820302. [DOI] [PubMed] [Google Scholar]
- Mizoguchi I, Nakamura M, Takahashi I, Kagayama M, Mitani H. An immunohistochemical study of localization of type I and type II collagens in mandibular condylar cartilage compared with tibial growth plate. Histochemistry. 1990;93:593–599. doi: 10.1007/BF00272201. [DOI] [PubMed] [Google Scholar]
- Mizoguchi I, Takahashi I, Nakamura M, Sasano Y, Sato S, Kagayama M, et al. An immunohistochemical study of regional differences in the distribution of type I and type II collagens in rat mandibular condylar cartilage. Arch. Oral Biol. 1996;41:863–869. doi: 10.1016/s0003-9969(96)00021-0. [DOI] [PubMed] [Google Scholar]
- Mizoguchi I, Takahashi I, Sasano Y, Kagayama M, Mitani H. Localization of types I, II and III collagen and glycosaminoglycans in the mandibular condyle of growing monkeys: an immunohistochemical study. Anat. Embryol. (Berlin) 1997;195:127–135. doi: 10.1007/s004290050031. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr Matrix metalloproteinases. J. Biol. Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Ohashi N, Ejiri S, Hanada K, Ozawa H. Change in type I, II, and X collagen immunoreactivity of the mandibular condylar cartilage in a naturally aging rat model. J. Bone Miner. Metab. 1997;15:77–83. [Google Scholar]
- Planus E, Galiacy S, Matthay M, Laurent V, Gavrilovic J, Murphy G, et al. Role of collagenase in mediating in vitro alveolar epithelial wound repair. J. Cell Sci. 1999;112:243–252. doi: 10.1242/jcs.112.2.243. [DOI] [PubMed] [Google Scholar]
- Salo LA, Hoyland J, Ayad S, Kielty CM, Freemont A, Pirttiniemi P, et al. The expression of types X and VI collagen and fibrillin in rat mandibular condylar cartilage. Response to mastication forces. Acta Odontol. Scand. 1996;54:295–302. doi: 10.3109/00016359609003541. [DOI] [PubMed] [Google Scholar]
- Sasano Y, Zhu JX, Tsubota M, Takahashi I, Onodera K, Mizoguchi I, et al. Gene expression of MMP8 and MMP13 during embryonic development of bone and cartilage in the rat mandible and hind limb. J. Histochem. Cytochem. 2002;50:325–332. doi: 10.1177/002215540205000304. [DOI] [PubMed] [Google Scholar]
- Shingleton WD, Hodges DJ, Brick P, Cawston TE. Collagenase: a key enzyme in collagen turnover. Biochem. Cell Biol. 1996;74:759–775. doi: 10.1139/o96-083. [DOI] [PubMed] [Google Scholar]
- Silbermann M, Reddi AH, Hand AR, Leapman RD, Von der Mark K, Franzen A. Further characterization of the extracellular matrix in the mandibular condyle in neonatal mice. J. Anat. 1987;151:169–188. [PMC free article] [PubMed] [Google Scholar]
- Silbermann M, von der Mark K. An immunohistochemical study of the distribution of matrical proteins in the mandibular condyle of neonatal mice. I. Collagens. J. Anat. 1990;170:11–22. [PMC free article] [PubMed] [Google Scholar]
- Takahashi I, Mizoguchi I, Sasano Y, Saitoh S, Ishida M, Kagayama M, et al. Age-related changes in the localization of glycosaminoglycans in condylar cartilage of the mandible in rats. Anat. Embryol. (Berlin) 1996;194:489–500. doi: 10.1007/BF00185995. [DOI] [PubMed] [Google Scholar]
- Tuckermann JP, Pittois K, Partridge NC, Merregaert J, Angel P. Collagenase-3 (MMP-13) and integral membrane protein 2a (Itm2a) are marker genes of chondrogenic/osteoblastic cells in bone formation: sequential temporal, and spatial expression of Itm2a, alkaline phosphatase, MMP-13, and osteocalcin in the mouse. J. Bone Miner. Res. 2000;15:1257–1265. doi: 10.1359/jbmr.2000.15.7.1257. [DOI] [PubMed] [Google Scholar]
- Uusitalo H, Hiltunen A, Soderstrom M, Aro HT, Vuorio E. Expression of cathepsins B, H, K, L, and S and matrix metalloproteinases 9 and 13 during chondrocyte hypertrophy and endochondral ossification in mouse fracture callus. Calcif. Tissue Int. 2000;67:382–390. doi: 10.1007/s002230001152. [DOI] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Kawashima A, Nebelung W, Neumann W, Roessner A. Immunohistochemical analysis of several proteolytic enzymes as parameters of cartilage degradation. Pathol. Res. Pract. 1998;194:73–81. doi: 10.1016/S0344-0338(98)80073-3. [DOI] [PubMed] [Google Scholar]
- Werb Z, Chin JR. Extracellular matrix remodeling during morphogenesis. Ann. NY Acad. Sci. 1998;857:110–118. doi: 10.1111/j.1749-6632.1998.tb10111.x. [DOI] [PubMed] [Google Scholar]
- Wu CW, Tchetina EV, Mwale F, Hasty K, Pidoux I, Reiner A, et al. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J. Bone Miner. Res. 2002;17:639–651. doi: 10.1359/jbmr.2002.17.4.639. [DOI] [PubMed] [Google Scholar]
- Yamaza T, Goto T, Kamiya T, Kobayashi Y, Sakai H, Tanaka T. Study of immunoelectron microscopic localization of cathepsin K in osteoclasts and other bone cells in the mouse femur. Bone. 1998;23:499–509. doi: 10.1016/s8756-3282(98)00138-0. [DOI] [PubMed] [Google Scholar]
- Yocum S, Lopresti-Morrow L, Reeves L, Mitchell P. MMP-13 and MMP-1 expression in tissues of normal articular joints. Ann. NY Acad. Sci. 1999;878:583–586. doi: 10.1111/j.1749-6632.1999.tb07734.x. [DOI] [PubMed] [Google Scholar]